- Record: found
- Abstract: found
- Article: found
Resveratrol Inhibits LPS-Induced MAPKs Activation via Activation of the Phosphatidylinositol 3-Kinase Pathway in Murine RAW 264.7 Macrophage Cells
Read this article at
Abstract
Background
Resveratrol is a natural polyphenolic compound that has cardioprotective, anticancer and anti-inflammatory properties. We investigated the capacity of resveratrol to protect RAW 264.7 cells from inflammatory insults and explored mechanisms underlying inhibitory effects of resveratrol on RAW 264.7 cells.
Methodology/Principal Findings
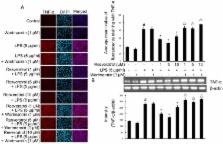
Murine RAW 264.7 cells were treated with resveratrol (1, 5, and 10 µM) and/or LPS (5 µg/ml). Nitric oxide (NO) and prostaglandin E2 (PGE2) were measured by Griess reagent and ELISA. The mRNA and protein levels of proinflammatory proteins and cytokines were analysed by ELISA, RT-PCR and double immunofluorescence labeling, respectively. Phosphorylation levels of Akt, cyclic AMP-responsive element-binding protein (CREB), mitogen-activated protein kinases (MAPKs) cascades, AMP-activated protein kinase (AMPK) and expression of SIRT1(Silent information regulator T1) were measured by western blot. Wortmannin (1 µM), a specific phosphatidylinositol 3-kinase (PI3-K) inhibitor, was used to determine if PI3-K/Akt signaling pathway might be involved in resveratrol’s action on RAW 264.7 cells. Resveratrol significantly attenuated the LPS-induced expression of nitric oxide (NO), prostaglandin E2 (PGE2), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in RAW 264.7 cells. Resveratrol increased Akt phosphorylation in a time-dependent manner. Wortmannin, a specific phosphatidylinositol 3-kinase (PI3-K) inhibitor, blocked the effects of resveratrol on LPS-induced RAW 264.7 cells activation. In addition, PI3-K inhibition partially abolished the inhibitory effect of resveratrol on the phosphorylation of cyclic AMP-responsive element-binding protein (CREB) and mitogen-activated protein kinases (MAPKs) cascades. Meanwhile, PI3-K is essential for resveratrol-mediated phosphorylation of AMPK and expression of SIRT1.
Conclusion and Implications
This investigation demonstrates that PI3-K/Akt activation is an important signaling in resveratrol-mediated activation of AMPK phosphorylation and SIRT1 expression, and inhibition of phosphorylation of CREB and MAPKs activation, proinflammatory mediators and cytokines production in response to LPS in RAW 264.7 cells.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3.
- Record: found
- Abstract: found
- Article: not found
Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer.
- Record: found
- Abstract: found
- Article: not found