- Record: found
- Abstract: found
- Article: not found
Synthesis and Ability of New Ligands for G Protein-Coupled Receptors 17 (GPR17)
Abstract
Background
GPR17 is believed to be a novel target for the development of new therapeutic approaches to human stroke and multiple sclerosis. Hence, the selection of GPR17 ligands may be a potent way to reduce the progression of ischemic damage.
Material/Methods
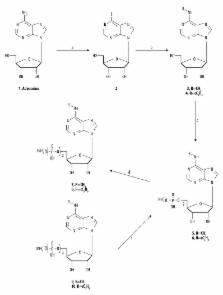
New potential ligands for GPR17, mono-, di-, and triphosphate adenosine nucleotides substituted at N6-position with a methyl and a cyclopentyl group were synthesized. The ability of new ligands to bind GPR17 was evaluated using frontal affinity chromatography-mass spectrometry (FAC-MS) method. Cangrelor, MRS2179, and uridine diphosphate were selected as the reference compounds.
Results
The new triphosphate derivatives 9 and 10 were considered as the new GPR17 ligands. The compound 10 was eluted with breakthrough time (bt) between cangrelor and MRS 2179 (compound 10, bt=12.25; cangrelor, bt=24.55, and MRS 2179, bt=7.10), while the breakthrough volume of compound 9 was similar to that of MRS 2179 (compound 9, bt=7.53 and MRS 2179, bt=7.10).
Related collections
Most cited references16
- Record: found
- Abstract: found
- Article: not found
The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor.
- Record: found
- Abstract: found
- Article: not found
The Recently Identified P2Y-Like Receptor GPR17 Is a Sensor of Brain Damage and a New Target for Brain Repair
- Record: found
- Abstract: found
- Article: not found
