- Record: found
- Abstract: found
- Article: found
GATA3 Expression Is Decreased in Psoriasis and during Epidermal Regeneration; Induction by Narrow-Band UVB and IL-4
Read this article at
Abstract
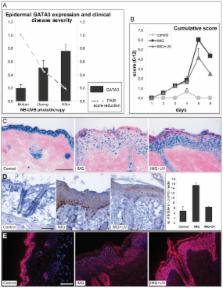
Psoriasis is characterized by hyperproliferation of keratinocytes and by infiltration of activated Th1 and Th17 cells in the (epi)dermis. By expression microarray, we previously found the GATA3 transcription factor significantly downregulated in lesional psoriatic skin. Since GATA3 serves as a key switch in both epidermal and T helper cell differentiation, we investigated its function in psoriasis. Because psoriatic skin inflammation shares many characteristics of epidermal regeneration during wound healing, we also studied GATA3 expression under such conditions.
Psoriatic lesional skin showed decreased GATA3 mRNA and protein expression compared to non-lesional skin. GATA3 expression was also markedly decreased in inflamed skin of mice with a psoriasiform dermatitis induced with imiquimod. Tape-stripping of non-lesional skin of patients with psoriasis, a standardized psoriasis-triggering and skin regeneration-inducing technique, reduced the expression of GATA3. In wounded skin of mice, low GATA3 mRNA and protein expression was detected. Taken together, GATA3 expression is downregulated under regenerative and inflammatory hyperproliferative skin conditions. GATA3 expression could be re-induced by successful narrow-band UVB treatment of both human psoriasis and imiquimod-induced psoriasiform dermatitis in mice. The prototypic Th2 cytokine IL-4 was the only cytokine capable of inducing GATA3 in skin explants from healthy donors. Based on these findings we argue that GATA3 serves as a key regulator in psoriatic inflammation, keratinocyte hyperproliferation and skin barrier dysfunction.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
DAVID: Database for Annotation, Visualization, and Integrated Discovery.
- Record: found
- Abstract: found
- Article: not found
Plasmacytoid predendritic cells initiate psoriasis through interferon-α production
- Record: found
- Abstract: found
- Article: not found