- Record: found
- Abstract: found
- Article: found
Impact of Genetic Variants on the Individual Potential for Body Fat Loss
Read this article at
Abstract
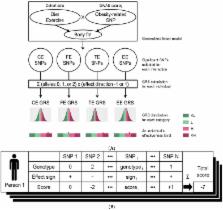
The past decade has witnessed the discovery of obesity-related genetic variants and their functions through genome-wide association studies. Combinations of risk alleles can influence obesity phenotypes with different degrees of effectiveness across various individuals by interacting with environmental factors. We examined the interaction between genetic variation and changes in dietary habits or exercise that influences body fat loss from a large Korean cohort ( n = 8840). Out of 673 obesity-related SNPs, a total of 100 SNPs (37 for carbohydrate intake; 19 for fat intake; 44 for total calories intake; 25 for exercise onset) identified to have gene-environment interaction effect in generalized linear model were used to calculate genetic risk scores (GRS). Based on the GRS distribution, we divided the population into four levels, namely, “very insensitive”, “insensitive”, “sensitive”, and “very sensitive” for each of the four categories, “carbohydrate intake”, “fat intake”, “total calories intake”, and “exercise”. Overall, the mean body fat loss became larger when the sensitivity level was increased. In conclusion, genetic variants influence the effectiveness of dietary regimes for body fat loss. Based on our findings, we suggest a platform for personalized body fat management by providing the most suitable and effective nutrition or activity plan specific to an individual.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma).
- Record: found
- Abstract: found
- Article: not found
15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma.
- Record: found
- Abstract: found
- Article: not found