- Record: found
- Abstract: found
- Article: found
A multi-mineral intervention to counter pro-inflammatory activity and to improve the barrier in human colon organoids
Read this article at
Abstract
Introduction: Ulcerative colitis is a chronic inflammatory condition, and continuous inflammatory stimulus may lead to barrier dysfunction. The goal of this study was to assess barrier proteomic expression by a red algae-derived multi-mineral intervention in the absence or presence of pro-inflammatory insult.
Methods: Human colon organoids were maintained in a control culture medium alone or exposed to lipopolysaccharide with a combination of three pro-inflammatory cytokines [tumor necrosis factor-α, interleukin-1β and interferon-γ (LPS-cytokines)] to mimic the environment in the inflamed colon. Untreated organoids and those exposed to LPS-cytokines were concomitantly treated for 14 days with a multi-mineral product (Aquamin ®) that has previously been shown to improve barrier structure/function. The colon organoids were subjected to proteomic analysis to obtain a broad view of the protein changes induced by the two interventions alone and in combination. In parallel, confocal fluorescence microscopy, tissue cohesion and transepithelial electrical resistance (TEER) measurements were used to assess barrier structure/function.
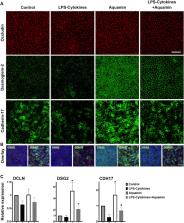
Results: The LPS-cytokine mix altered the expression of multiple proteins that influence innate immunity and promote inflammation. Several of these were significantly decreased with Aquamin ® alone but only a modest decrease in a subset of these proteins was detected by Aquamin ® in the presence of LPS-cytokines. Among these, a subset of inflammation-related proteins including fibrinogen-β and -γ chains (FGB and FGG), phospholipase A2 (PLA2G2A) and SPARC was significantly downregulated in the presence of Aquamin ® (alone and in combination with LPS-cytokines); another subset of proteins with anti-inflammatory, antioxidant or anti-microbial activity was upregulated by Aquamin ® treatment. When provided alone, Aquamin ® strongly upregulated proteins that contribute to barrier formation and tissue strength. Concomitant treatment with LPS-cytokines did not inhibit barrier formation in response to Aquamin ®. Confocal microscopy also displayed increased expression of desmoglein-2 (DSG2) and cadherin-17 (CDH17) with Aquamin ®, either alone or in the presence of the pro-inflammatory stimulus. Increased cohesion and TEER with Aquamin ® (alone or in the presence of LPS-cytokines) indicates improved barrier function.
Conclusion: Taken together, these findings suggest that multi-mineral intervention (Aquamin ®) may provide a novel approach to combating inflammation in the colon by improving barrier structure/function as well as by directly altering the expression of certain pro-inflammatory proteins.
Related collections
Most cited references71
- Record: found
- Abstract: found
- Article: not found
MultiNotch MS3 Enables Accurate, Sensitive, and Multiplexed Detection of Differential Expression across Cancer Cell Line Proteomes
- Record: found
- Abstract: found
- Article: not found
Tight junctions: from simple barriers to multifunctional molecular gates.

- Record: found
- Abstract: found
- Article: found