- Record: found
- Abstract: found
- Article: found
Respiratory Effects of Treatment with a Glucagon-Like Peptide-1 Receptor Agonist in Patients Suffering from Obesity and Chronic Obstructive Pulmonary Disease
Abstract
Purpose
Chronic obstructive pulmonary disease (COPD) affects millions of people worldwide. Obesity is commonly seen concomitantly with COPD. People with COPD have reduced quality of life, reduced physical activity, chronic respiratory symptoms, and may suffer from frequent clinical exacerbations. Liraglutide is a glucagon-like peptide-1 receptor agonist (GLP-1RA) approved for weight loss and treatment of type-2 diabetes mellitus. In addition, liraglutide exerts anti-inflammatory actions by reducing IL-6 and MCP-1 levels. We investigated the effect of liraglutide on pulmonary function in people suffering from obesity and COPD.
Patients and Methods
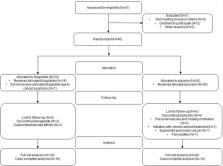
In this controlled, double-blind trial, 40 people with obesity and COPD from two outpatient clinics were allocated randomly to receive liraglutide (3.0 mg, s.c.) or placebo (s.c.) for 40 weeks. At baseline and after 4, 20, 40, and 44 weeks, participants underwent pulmonary-function tests, 6-min walking test, and replied to a questionnaire regarding the clinical impact of COPD (COPD assessment test (CAT)-score).
Results
Compared with placebo, liraglutide use resulted in significant weight loss, increased forced vital capacity (FVC) and carbon monoxide diffusion capacity, and improved CAT-score. We found no significant changes in forced expiratory volume in one second (FEV 1), FEV 1/FVC, or 6-min walking distance.
Most cited references28
- Record: found
- Abstract: found
- Article: not found
A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management.
- Record: found
- Abstract: found
- Article: not found
The physiology of glucagon-like peptide 1.
- Record: found
- Abstract: found
- Article: not found