- Record: found
- Abstract: found
- Article: found
miR-93 Promotes Cell Proliferation in Gliomas through Activation of PI3K/Akt Signaling Pathway
Read this article at
Abstract
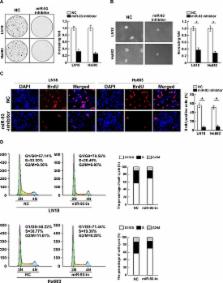
The PI3K/Akt signaling pathway is frequently activated in various human cancer types and plays essential roles in development and progression of cancers. Multiple regulators, such as phosphatase and tensin homolog (PTEN) and PH domain leucine rich repeat protein phosphatases (PHLPP), have also found to be involved in suppression of the PI3K/Akt signaling pathway. However, how suppressive effects mediated by these regulators are concomitantly disrupted in cancers, which display constitutively activated PI3K/Akt signaling, remains puzzling. In the present study, we reported that the expression of miR-93 was markedly upregulated in glioma cell lines and clinical glioma tissues. Statistical analysis revealed that miR-93 levels significantly correlated with clinicopathologic grade and overall survival in gliomas. Furthermore, we found that overexpressing miR-93 promoted, but inhibition of miR-93 reduced, glioma cell proliferation and cell-cycle progression. We demonstrated that miR-93 activated PI3K/Akt signaling through directly suppressing PTEN, PHLPP2 and FOXO3 expression via targeting their 3′UTRs. Therefore, our results suggest that miR-93 might play an important role in glioma progression and uncover a novel mechanism for constitutive PI3K/Akt activation in gliomas.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal.
- Record: found
- Abstract: not found
- Article: not found
AKT plays a central role in tumorigenesis.
- Record: found
- Abstract: found
- Article: not found