- Record: found
- Abstract: found
- Article: found
In situ delivery of thermosensitive gel-mediated 5-fluorouracil microemulsion for the treatment of colorectal cancer
Abstract
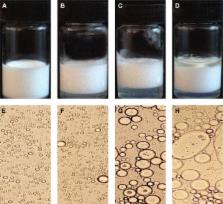
In situ administration of 5-fluorouracil (5FU) “thermosensitive” gel effectively reduced systemic side effects in treating colon rectal cancer; however, the penetration efficacy of the formulation was considerably low due to the poor lipid solubility of 5FU. The aim of this study was to develop thermosensitive gel-mediated 5FU water-in-oil microemulsion (TG-5FU-ME) for improving the infiltration of 5FU. An in vitro release test showed that TG-5FU-ME sustained the drug’s release up to 10 hours. TG-5FU-ME exhibited good stability, and the microemulsion entrapped did not show any change in morphology and 5FU content during the 4-month storage. Transportation test in the Caco-2 cell monolayer showed that TG-5FU-ME had a permeability 6.3 times higher than that of 5FU thermosensitive gel, and the intracellular uptake of 5FU increased by 5.4-fold compared to that of 5FU thermosensitive gel. In vivo tissue distribution analysis exhibited that the TG-5FU-ME group had drug levels in rectal tissue and mesenteric lymph nodes, which were significantly higher than those of 5FU thermosensitive gel group, with very low blood levels of 5FU in both groups. Furthermore, TG-5FU-ME was not associated with detectable morphological damage to the rectal tissue. Conclusively, TG-5FU-ME might be an efficient rectal delivery system to treat colorectal cancer.
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Thermosensitive sol-gel reversible hydrogels.
- Record: found
- Abstract: found
- Article: not found
In vitro combinatorial anticancer effects of 5-fluorouracil and curcumin loaded N,O-carboxymethyl chitosan nanoparticles toward colon cancer and in vivo pharmacokinetic studies.
- Record: found
- Abstract: found
- Article: not found