- Record: found
- Abstract: found
- Article: found
Synergistic enzymatic and bioorthogonal reactions for selective prodrug activation in living systems
Read this article at
Abstract
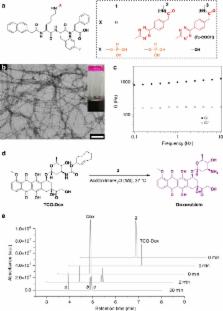
Adverse drug reactions (ADRs) restrict the maximum doses applicable in chemotherapy, which leads to failure in cancer treatment. Various approaches, including nano-drug and prodrug strategies aimed at reducing ADRs, have been developed, but these strategies have their own pitfalls. A renovated strategy for ADR reduction is urgently needed. Here, we employ an enzymatic supramolecular self-assembly process to accumulate a bioorthogonal decaging reaction trigger inside targeted cancer cells, enabling spatiotemporally controlled, synergistic prodrug activation. The bioorthogonally activated prodrug exhibits significantly enhanced potency against cancer cells compared with normal cells. This prodrug activation strategy further demonstrates high tumour inhibition efficacy with satisfactory biocompatibility, pharmacokinetics, and safety in vivo. We envision that integration of enzymatic and bioorthogonal reactions will serve as a general small-molecule-based strategy for alleviation of ADRs in chemotherapy.
Abstract
The side effects of cancer drugs limit their utility. Here, the authors developed a method in which an inactive (prodrug) version of the cancer drug doxorubicin enters tumour cells and then gets activated inside the cells upon a trigger facilitated by enzyme-instructed supramolecular self-assembly.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers.
- Record: found
- Abstract: found
- Article: not found
Bioorthogonal Cyclization-Mediated In Situ Self-Assembly of Small Molecule Probes for Imaging Caspase Activity in vivo
- Record: found
- Abstract: found
- Article: not found