- Record: found
- Abstract: found
- Article: found
Standardized Outcomes in Nephrology-Transplantation: A Global Initiative to Develop a Core Outcome Set for Trials in Kidney Transplantation
Abstract
Background
Although advances in treatment have dramatically improved short-term graft survival and acute rejection in kidney transplant recipients, long-term graft outcomes have not substantially improved. Transplant recipients also have a considerably increased risk of cancer, cardiovascular disease, diabetes, and infection, which all contribute to appreciable morbidity and premature mortality. Many trials in kidney transplantation are short-term, frequently use unvalidated surrogate endpoints, outcomes of uncertain relevance to patients and clinicians, and do not consistently measure and report key outcomes like death, graft loss, graft function, and adverse effects of therapy. This diminishes the value of trials in supporting treatment decisions that require individual-level multiple tradeoffs between graft survival and the risk of side effects, adverse events, and mortality. The Standardized Outcomes in Nephrology-Transplantation initiative aims to develop a core outcome set for trials in kidney transplantation that is based on the shared priorities of all stakeholders.
Methods
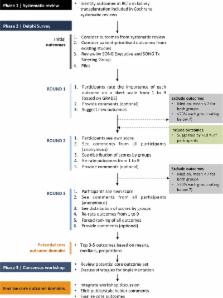
This will include a systematic review to identify outcomes reported in randomized trials, a Delphi survey with an international multistakeholder panel (patients, caregivers, clinicians, researchers, policy makers, members from industry) to develop a consensus-based prioritized list of outcome domains and a consensus workshop to review and finalize the core outcome set for trials in kidney transplantation.
Conclusions
Developing and implementing a core outcome set to be reported, at a minimum, in all kidney transplantation trials will improve the transparency, quality, and relevance of research; to enable kidney transplant recipients and their clinicians to make better-informed treatment decisions for improved patient outcomes.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: found
Developing core outcome sets for clinical trials: issues to consider
- Record: found
- Abstract: found
- Article: not found
Long-term renal allograft survival in the United States: a critical reappraisal.
- Record: found
- Abstract: found
- Article: not found