- Record: found
- Abstract: found
- Article: found
Structured assessment of frailty in multiple myeloma as a paradigm of individualized treatment algorithms in cancer patients at advanced age
research-article
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Introduction
Life expectancy and incidence of cancer have substantially increased, the latter being
closely interlinked to our longevity. Today, 617 million people are ≥65 years; by
2050, this number will have reached 1.6 billion, nearly 20% of the world’s population,
and the number of "very old" (>80 years) will have more than tripled.
1
This aging of the population involves enormous changes to patient care. For the moment,
the most profound changes are to be seen in Japan, Europe and North America. Major
risk factors associated with aging include cancer (also multiple cancers in a single
patient),
2
and cardiovascular and neurodegenerative diseases, all requiring long-term care. Therefore,
especially high-income countries are obliged to meet the challenges.
1
Multiple myeloma (MM), as one example of cancer, and the 2nd most frequent hematologic
malignancy, affects adults of all ages, but is primarily a disease of the elderly.
The highest burden of MM-related deaths occurs among persons between 65 and 84 years
of age.
3–7
Similarly to the situation in several other types of cancers, management of older
MM patients is more demanding due to their often impaired organ function, underlying
comorbidities, and co-existing frailty, which may increase therapy-related toxicity,
and lead to dose reduction and shorter treatment endurance.
3,4,6–9
The high prevalence of geriatric impairments is increasingly being recognized, but
is not always easily detectable without an objective assessment.
3,6,7
Our goal today involves reducing the risk of under-treating fit patients and over-treating
those who are frail.
5,10–12
Although eligibility criteria for studies of anti-cancer/-MM agents have traditionally
relied on age cut-offs and performance status, geriatric and MM-specific frailty assessments
are just beginning to be incorporated into more accurate stratification plans of treatment
algorithms.
6,7,11,12
Similarly to MM patients, geriatric assessments (GA) have been defined for patients
with chronic lymphocytic leukemia (CLL)
8,13,14
and myelodysplastic syndrome (MDS),
15,16
where determination of frailty versus fitness has moved into clinical practice. However,
solutions as to how they might be more uniformly used and valued in their daily pratice
have not been fully determined.
Recommendations of the geriatric oncology working groups (i.e. German Society of Geriatrics/German
Society of Hematoloogy&Oncology) have suggested GA-tools to check comorbidity in patients
aged ≥70 years via the Charlson Comorbidity Index (CCI), cognition via the Mini Mental
test (MMST), activity/instrumental activity of daily living (ADL/IADL), mobility via
the Timed Up and Go test, depression via the geriatric depression scale (GDS), and
nutrition via body mass index (BMI) and Mini Nutritional Status.
6,7,11,12,17
While these GA-tools have been established and validated, their execution is time-consuming,
an additional workforce is needed, and the involvement of a geriatric team is advisable.
6,7,9,11,12,17
Whether shorter frailty scores in cancer patients may substitute and/or add to GA-tools
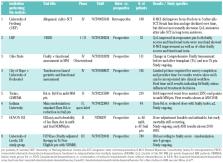
is being pursued in single- and multi-center trials (Table 1).
Table 1
Selected clinical trials in multiple myeloma patients with frailty assessments included
therein.
The aim of this commentary is to define strategies in MM patients, and explore how
frailty assessment may be employed in clinical practice and clinical trials.
Instruments to assess vulnerability due to increased treatment options
The epidemiologic and biologic considerations of elderly MM patients, with widely
expanding treatment options, have motivated global efforts to validate simple instruments
to assess vulnerability of patients, test them in their clinical significance to predict
treatment outcome [overall survival (OS) and progression-free survival (PFS)], occurrence
of severe adverse events, and to tailor treatment with more or less intensified regimens.
11,12,18
Under-treatment of fit elderly patients has been demonstrated to occur more frequently
than over-treatment.
12
Under-treatment may prevent improvement of organ function, while over-treatment of
frail patients can induce unnecessary morbidity and mortality. Both instances reduce
patients’ health-related quality of life (HRQOL). In a study that assessed HRQOL across
>16,000 cancer survivors, those with MM were among those with the lowest HRQOL scores,
highlighting the urgent need for this to be improved and for frequent reassessment
of HRQOL in cancer patients.
19
The art of managing elderly MM patients involves balancing competing disease-related
and patient-specific factors and to make adequate treatment decisions.
Numerous induction (and relapse) MM-treatment options are available today. These include
bortezomib-cyclophosphamide-dexamethasone (VCD), bortezomib-lenalidomide-dexamethasone
(VRD or VTD), bortezomib-melphalan-prednisone (VMP) or antibody-combinations, autologous
stem cell transplantation (ASCT) and 2-drug combinations, such as lenalidomide-dexamethasone
(Rd), bortezomib-dexamethasone (Vd), and others.
3,20–22
These largely expanded therapeutic strategies, including immunotherapies,
23
have significantly evolved in recent years, but the beneficial effect is not seen
across the age spectrum, with intermediate-fit or frail patients not obtaining the
maximal benefit from such new treatment. Part of this failure to achieve benefit relates
to the host biology of older patients. Therefore, there is an unmet need to give the
right therapy to the patient most suited to benefit from it; the starting point for
this approach is an appropriate classification of who is fit and who is frail.
Risk parameters in multiple myeloma
That age alone is a much less well-suited discriminator for treatment designation
has been shown via various risk parameters and comorbidity scores, that are usually
described as patient-related factors.
11,12,24–26
These involve simple measures of daily activity, such as constitution via Eastern
Cooperative Oncology Group (ECOG) or Karnofsky performance status (KPS), organ function,
and comorbidities. However, because the KPS/ECOG do not reflect the entire functional
status of cancer patients, advances in defining patient fitness more precisely are
warranted. In an analysis of 466 consecutive MM patients, the median KPS was determined
to be 90%, although a precise reassessment showed this was actually 60%, i.e. 30%
lower than that estimated by physicians. This clearly demonstrates that both KPS/ECOG
are often over-estimated, and a more precise frailty assessment is valuable.
25
In a subsequent analysis, 13 comorbid conditions were assessed in 801 patients. These
were graded and rated according to the Common Terminology Criteria for Adverse Events
(CTCAE 4.03), which included: renal-, lung- and KPS-impairment, cardiac, liver or
gastrointestinal disease, disability, frailty, infection, thromboembolic events, peripheral
neuropathy, pain, and secondary malignancies. In addition, age, cytogenetics via fluorescence
in situ hybridization, renal function and lung disease were determined. The multivariate
Cox proportional hazard model based on backward selection revealed five highly significant
risks as relevant for OS: renal and lung function, KPS, age, and frailty (Fried definition).
Score weights for comorbidities were determined on the basis of regression coefficients
of the prognostic factors.
12
Although impairment of organ function such as lung disease had been defined as having
nothing to do with MM, in line with other large MM study groups (such as both the
German GMMG and DSMM study groups), patients with moderate and severe lung impairment
and continued smoking habit were at substantial risk for treatment complications.
11,12
We would, therefore, refrain from ASCT/allogeneic-SCT, triplet and quadruplet therapies
in heavy smokers and/or those with impaired lung function.
11,12,24–26
Moreover, disease-related factors add additional complexity in MM, such as cytogenetics,
International Staging System (ISS)/revised (R)-ISS stage, bone marrow infiltration,
and number of CRAB (C, hypercalcemia; R, renal impairment; A, anemia; B, bone lesions)
symptoms. In addition, treatment-related factors, such as how quickly and for how
long the disease responds to therapy, are critical.
27–29
Frailty, organ impairment and myeloma scores
In prior organ function analyses,
11,12,24–26,30
the extent of frailty in MM patients was substantial: 60% for entire (mild to severe)
and 40% for severe frailty.
11,12
This led to the development of the revised myeloma comorbidity score (R-MCI). This
R-MCI uses weights generated via multivariate risk factor assessment with the essential
risks being included therein, such as: renal and lung function, KPS, frailty and age,
with the option to add cytogenet-ics.
11,12
Apart from organ impairment, cytogenetic aberrations corroborate with impaired OS
in MM patients. The analysis confirmed that cytogenetics provide independent additional
information,
31–35
and that patients with unfavorable cytogenetics had higher disease stages, adverse
laboratory values, and reduced organ and physical function. Although cytogenetics
proved to be a relevant risk factor, the analysis confirmed that others, such as physical
and organ conditions, are equally important.
6,7,11,12,18
Moreover, development of the R-MCI showed that the multivariate risks (renal, lung
function, KPS, age, frailty) defined patients as fit, intermediate-fit, and frail,
which could be improved with inclusion of cytogenetics, (but which could still be
used even if this information was unavailable). Weighting of the R-MCI verified that
this 9-point score defines three patient groups with clearly different survival,
12
which remained true regardless of treatment or age subgroups.
Comparison of the R-MCI with others and current questions
Comparison of the R-MCI with numerous others [CCI, Kaplan Feinstein (KF), Hematopoietic
Cell Transplantation Comorbidity Index (HCT-CI/Sorer),
12
the Satariano Index
24–26
or the International Myeloma Working Group (IMWG frailty score
11
) showed that they all divide patients into risk groups with substantially different
OS. However, Brier scores determined the smallest prediction errors with the R-MCI.
One reason for the comparability of the R-MCI with others was that most include risks
that have some relevance in MM, namely renal and lung function, and physical condition.
Compared to the initial non-weighted MCI,
24–26
the R-MCI led to an improvement in group distinction, which highlights the relevance
to further improve a risk score, as performed in subsequent analyses.
11,12,24–26,30
Various risk scores that are used in different institutions and within clinical trials
in MM patients are summarized in Table 1.
18,36–38
The question is, therefore, whether one comorbidity score in MM should be put forward
or whether more should be developed. Moreover, would harmonization and inclusion of
biomarkers improve them?
39
Another question is if MM experts will use these scores and whether treatment decisions
are being improved.
40,41
Whether risks determined by a score result in changes in treatment decision has not
been fully addressed. Given that MM primarily affects the elderly, whose vulnerabilities
may change over time, it is also reasonable to incorporate serial GA throughout treatment
in order to potentially modify therapy over time and incorporate this into tumorboards
and treatment guidelines. For older, fit patients, intensive treatment with ASCT may
be appropriate, while in the very frail, with GA and high R-MCI-scores, end-of-life
care discussions can be facilitated.
10,42
Concrete clinical designations of the use of the R-MCI
We have included the R-MCI in the weekly MM tumorboard, where this is being scored
before the patient is discussed at an interdisciplinary level. Web applications make
it easy to obtain a score end result, as has been achieved for the R-MCI and IMWG-frailty
scores.
11,12,18,40
For the R-MCI, each patient’s individual risk parameters will generate an R-MCI score.
Training and validation analyses of the R-MCI showed well-discriminated risk profiles
in terms of both PFS and OS for fit, intermediate fit, and frail patients.
12
This was true both for more intensively and less intensively treated MM patients.
12
Moreover, if MM patients were risk-assessed via the R-MCI rather than the IMWG-frailty
score, Kaplan-Meier analysis produced more clearly separated PFS and OS curves with
the the R-MCI than with the IMWG-frailty score.
11
Importantly, if patients are intermediate-fit or frail by R-MCI, precautions for dose
reduction of systemic treatment can be made: i.e. if advanced frailty in MM patients
is observed, dose reductions can be discussed, including whether the disease aggressiveness
needs effective anti-MM treatment to be performed in spite of the patient’s frailty.
Today, it seems important, given the widely different anti-MM treatment options, that
the frailty scoring specifically warns MM experts that complications with intensive
treatment may occur. As many precautions as possible can then be taken while treatment
is being given, such as inpatient rather than outpatient treatment, observation on
the ward until complications no longer occur, prophylactic medications, etc. In line
with this, in their joint EMN-paper,
7
the European Myeloma Network (EMN) consensus has stated that in fit MM patients, efficient
antimyeloma therapy with the aim of deep remission is key, whereas in unfit or frail
patients, the priority is to maintain a good balance between therapy efficacy and
safety.
Useful dose adaptations have been recommended for individual antimyeloma agents and
are published as such in guidelines and chemotherapy manuals.
6,7,43
The R-MCI has also been included in study protocols before therapy initiation and
at the end of treatment. This can assess whether a patient’s constitution did improve
over time, and whether this was associated with myeloma response and better functional
comorbidity tests (Table 1).
21,44
The R-MCI has, indeed, allowed a patient’s improved constitution to be demonstrated;
this has also been assessed in rarer treatment scenarios, such as in younger, high-risk
patients undergoing immunotherapy approaches, i.e. allo-SCT. Here, although patients
grew older and renal function declined over time, the median R-MCI improved from 4
before allo-SCT to 3 after allo-SCT (Table 1).
23,45
In frail patients, being able to see if there is any deterioration in the R-MCI makes
it easier to adapt or interrupt treatment. This underscores its clinical helpfulness.
For example, since the QoL in a light chain (AL)-amyloidosis patient did not improve,
even though hematologic response was achieved, the use of the R-MCI facilitated supportive
treatment rather than continuation of extensive and expensive care.
10,46
Inclusion of the R-MCI in future study protocols at our center, and in discussion
with both German MM study groups (DSMM and GMMG) is under way.
Conclusions
Although the IMWG-frailty score is a “reference” comorbidity index,
18
others are more straightforward to use. The inclusion of “Lung function” in the R-MCI
had been repeatedly requested by reviewers as a more objective measure than via the
GOLD criteria, smoking status or dyspnea upon exertion, and is included in the diagnostic
workup at our center (i.e. before intensive treatment, such as SCT).
11,12,21,44–47
If unavailable, smoking status, its mandatory cessation before SCT/intensive treatment,
no advanced GOLD criteria, and no dyspnea upon exertion have been used as substitutes
in prior analyses.
24–26
We have demonstrated the validity of the R-MCI as a valid prognostic instrument in
large MM cohorts treated according to current standards. Based on existing recommendations,
the R-MCI can be applied in routine clinical care, multicenter analyses and future
clinical trials. It may be used in research to compare risk profiles of MM cohorts,
to adjust for imbalanced risks, and to provide a basis to establish new clinical or
biologic prognostic factors. Moreover, the R-MCI might be considered to be an integral
part in the development of individualized risk-adapted treatment strategies to further
improve outcome in MM. This includes correct use of resources, higher inclusion rates
of older patients into clinical studies, and avoidance of under-supply for older but
fit patients.
In the future, the R-MCI could also help to support treatment decisions, tolerability,
and to avoid toxicity. Since any prospective comorbidity, frailty and disability evaluation
can be time-consuming, we have implemented the R-MCI within a web-based technology
application which allows a quick turnaround of results.
48
Routine measurement of frailty in MM patients is, therefore feasible, and several
analyses via R-MCI
11,12
(or IMWG frailty scores
18
with various adaptations
6,47
) are available.
All current developments in the field are enthusiastically welcomed, because more
effective and individualized treatment offers an opportunity to further improve clinical
outcomes, especially among older patients with hematologic malignancies.
21,27,28,49
We have proven tools for a functional, more objective assessment to help guide every-day
treatment, and these should be incorporated into tumor boards and may allow better
trial comparability, as well as helping to guide trial design. It will be interesting
to see whether, in the near future, these risk tools are readily implemented into
clinical care and can improve patient management. We are entering an exciting era
for research on aging, which holds unprecedented promise for increased patient lifespan,
delaying pathologies of aging, allowing patients to grow old, and living a life full
of purpose and well-being (Figure 1).
1,50
Future clinical trials that target the aging process and that study biomarkers and
intervention programs face considerable challenges, but the potential rewards will
far outweigh their risks.
1,50
Figure 1
Environmental and genetic factors that influence key cellular processes and pathways
defined as hallmarks of aging. Many pathways contribute to the creation of a chronic
inflammatory stage and to aging. These in turn increase the risk of chronic disease
of aging together with disease-specific risk factors, i.e. in multiple myeloma (MM):
polyneuropathy, osteoporosis/osteopenia, bone fractures, anemia. All eventually induce
frailty, disability, mortality and geriatric syndromes, and potentially decrease patients’
quality of life (adapted and with permission of J. Campisi et al.)
50
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
From discoveries in ageing research to therapeutics for healthy ageing
Judith Campisi, Pankaj Kapahi, Gordon Lithgow … (2019)
- Record: found
- Abstract: found
- Article: not found
Combining fluorescent in situ hybridization data with ISS staging improves risk assessment in myeloma: an International Myeloma Working Group collaborative project.
O Sezer, B Barlogie, R Fonseca … (2013)
