- Record: found
- Abstract: found
- Article: not found
Evidence against dopamine D1/D2 receptor heteromers
Read this article at
Abstract
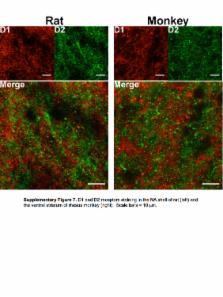
Hetero-oligomers of G-protein-coupled receptors have become the subject of intense investigation because their purported potential to manifest signaling and pharmacological properties that differ from the component receptors makes them highly attractive for the development of more selective pharmacological treatments. In particular, dopamine D1 and D2 receptors have been proposed to form hetero-oligomers that couple to G αq proteins, and SKF83959 has been proposed to act as a biased agonist that selectively engages these receptor complexes to activate G αq and thus phospholipase C. D1/D2 heteromers have been proposed as relevant to the pathophysiology and treatment of depression and schizophrenia. We used in vitro bioluminescence resonance energy transfer (BRET), ex vivo analyses of receptor localization and proximity in brain slices, and behavioral assays in mice to characterize signaling from these putative dimers/oligomers. We were unable to detect G αq or G α11 protein coupling to homomers or heteromers of D1 or D2 receptors using a variety of biosensors. SKF83959-induced locomotor and grooming behaviors were eliminated in D 1 receptor knockout mice, verifying a key role for D1-like receptor activation. In contrast, SKF83959-induced motor responses were intact in D2 receptor and G αq knockout mice, as well as in knock-in mice expressing a mutant Ala 286-CaMKIIα, that cannot autophosphorylate to become active. Moreover, we found that in the shell of the nucleus accumbens, even in neurons in which D1 and D2 receptor promoters are both active, the receptor proteins are segregated and do not form complexes. These data are not compatible with SKF83959 signaling through G αq or through a D1–D2 heteromer and challenge the existence of such a signaling complex in the adult animals that we used for our studies.
Related collections
Most cited references68
- Record: found
- Abstract: found
- Article: not found
A gene expression atlas of the central nervous system based on bacterial artificial chromosomes.
- Record: found
- Abstract: found
- Article: not found
Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning.
- Record: found
- Abstract: found
- Article: not found
