- Record: found
- Abstract: found
- Article: found
Biomimetic collagenous scaffold to tune inflammation by targeting macrophages
Read this article at
Abstract
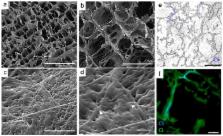
The inflammatory response following implantation of a biomaterial is one of the major regulatory aspects of the overall regenerative process. The progress of inflammation determines whether functional tissue is restored or if nonfunctional fibrotic tissue is formed. This delicate balance is directed by the activity of different cells. Among these, macrophages and their different phenotypes, the inflammatory M1 to anti-inflammatory M2, are considered key players in the process. Recent approaches exploit macrophage’s regenerative potential in tissue engineering. Here, we propose a collagen scaffold functionalized with chondroitin sulfate (CSCL), a glycosaminoglycan known to be able to tune inflammation. We studied CSCL effects on bone-marrow-derived macrophages in physiological, and lipopolysaccharides-inflamed, conditions in vitro. Our data demonstrate that CSCL is able to modulate macrophage phenotype by inhibiting the LPS/CD44/NF-kB cascade. As a consequence, an upregulation of anti-inflammatory markers ( TGF-β, Arg, MRC1, and IL-10) was found concomitantly with a decrease in the expression of pro-inflammatory markers ( iNOS, TNF-α, IL-1β, IL-12β). We then implanted CSCL subcutaneously in a rat model to test whether the same molecular mechanism could be maintained in an in vivo environment. In vivo data confirmed the in vitro studies. A significant reduction in the number of infiltrating cells around and within the implants was observed at 72 h, with a significant downregulation of pro-inflammatory genes expression. The present work provides indications regarding the immunomodulatory potential of molecules used for the development of biomimetic materials and suggests their use to direct macrophage immune modulation for tissue repair.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Modulating the stem cell niche for tissue regeneration.
- Record: found
- Abstract: found
- Article: not found
Macrophage phenotype as a determinant of biologic scaffold remodeling.
- Record: found
- Abstract: found
- Article: not found