- Record: found
- Abstract: found
- Article: found
TTC7A: Steward of Intestinal Health
Read this article at
Abstract
The increasing incidence of pediatric inflammatory bowel disease, coupled with the efficiency of whole-exome sequencing, has led to the identification of tetratricopeptide repeat domain 7A (TTC7A) as a steward of intestinal health. TTC7A deficiency is an autosomal-recessively inherited disease. In the 5 years since the original description, more than 50 patients with more than 20 distinct disease-causing TTC7A mutations have been identified. Patients show heterogenous intestinal and immunologic disease manifestations, including but not limited to multiple intestinal atresias, very early onset inflammatory bowel disease, loss of intestinal architecture, apoptotic enterocolitis, combined immunodeficiency, and various extraintestinal features related to the skin and/or hair. The focus of this review is to highlight trends in patient phenotypes and to consolidate functional data related to the role of TTC7A in maintaining intestinal homeostasis. TTC7A deficiency is fatal in approximately two thirds of patients, and, as more patients continue to be discovered, elucidating the comprehensive role of TTC7A could show druggable targets that may benefit the growing cohort of individuals suffering from inflammatory bowel disease.
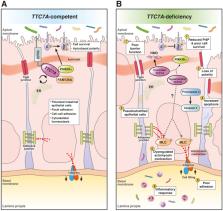
Graphical abstract
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease
- Record: found
- Abstract: found
- Article: not found
The Intestinal Epithelium: Central Coordinator of Mucosal Immunity
- Record: found
- Abstract: found
- Article: not found
