- Record: found
- Abstract: found
- Article: found
Emerging Roles of Sympathetic Nerves and Inflammation in Perivascular Adipose Tissue
Read this article at
Abstract
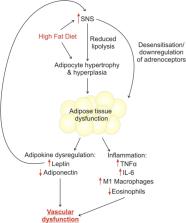
Perivascular adipose tissue (PVAT) is no longer recognised as simply a structural support for the vasculature, and we now know that PVAT releases vasoactive factors which modulate vascular function. Since the discovery of this function in 1991, PVAT research is rapidly growing and the importance of PVAT function in disease is becoming increasingly clear. Obesity is associated with a plethora of vascular conditions; therefore, the study of adipocytes and their effects on the vasculature is vital. PVAT contains an adrenergic system including nerves, adrenoceptors and transporters. In obesity, the autonomic nervous system is dysfunctional; therefore, sympathetic innervation of PVAT may be the key mechanistic link between increased adiposity and vascular disease. In addition, not all obese people develop vascular disease, but a common feature amongst those that do appears to be the inflammatory cell population in PVAT. This review will discuss what is known about sympathetic innervation of PVAT, and the links between nerve activation and inflammation in obesity. In addition, we will examine the therapeutic potential of exercise in sympathetic stimulation of adipose tissue.
Related collections
Most cited references150
- Record: found
- Abstract: found
- Article: not found
Plasma adiponectin levels and risk of myocardial infarction in men.
- Record: found
- Abstract: found
- Article: not found
Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms.
- Record: found
- Abstract: found
- Article: not found