- Record: found
- Abstract: found
- Article: found
Third‐line antiretroviral therapy, including raltegravir (RAL), darunavir (DRV/r) and/or etravirine (ETR), is well tolerated and achieves durable virologic suppression over 144 weeks in resource‐limited settings: ACTG A5288 strategy trial
Read this article at
Abstract
Introduction
ACTG A5288 was a strategy trial conducted in diverse populations from multiple continents of people living with HIV (PLWH) failing second‐line protease inhibitor (PI)‐based antiretroviral therapy (ART) from 10 low‐ and middle‐income countries (LMICs). Participants resistant to lopinavir (LPV) and/or multiple nucleotide reverse transcriptase inhibitors started on third‐line regimens that included raltegravir (RAL), darunavir/ritonavir (DRV/r) and/or etravirine (ETR) according to their resistance profiles. At 48 weeks, 87% of these participants achieved HIV‐1 RNA ≤200 copies/ml. We report here long‐term outcomes over 144 weeks.
Methods
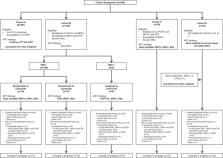
Study participants were enrolled from 2013 to 2015, prior to the availability of dolutegravir in LMICs. “Extended Follow‐up” of the study started after the last participant enrolled had reached 48 weeks and included participants still on antiretroviral (ARV) regimens containing RAL, DRV/r and/or ETR at that time. RAL, DRV/r and ETR were provided for an additional 96 weeks (giving total follow‐up of ≥144 weeks), with HIV‐1 RNA measured at 48 and 96 weeks and CD4 count at 96 weeks after entry into Extended Follow‐up. Proportion of participants with HIV‐1 RNA ≤200 copies/ml was estimated every 24 weeks, using imputation if necessary to handle the different measurement schedule in Extended Follow‐up; mean CD4 count changes were estimated using loess regression.
Results and Discussion
Of 257 participants (38% females), at study entry, median CD4 count was 179 cells/mm 3, and HIV‐1 RNA was 4.6 log 10 copies/ml. Median follow‐up was 168 weeks (IQR: 156–204); 15 (6%) participants were lost to follow‐up and 9 (4%) died. 27/246 (11%), 26/246 (11%) and 13/92 (14%) of participants who started RAL, DRV/r and ETR, respectively, discontinued these drugs; only three due to adverse events. 87%, 86%, 83% and 80% of the participants had HIV‐1 RNA ≤200 copies/ml at weeks 48, 96, 144 and 168 (95% CI at week 168: 74–85%), respectively. Mean increase from study entry in CD4 count at week 168 was 265 cells/mm 3 (95% CI 247–283).
Related collections
Most cited references15
- Record: found
- Abstract: found
- Article: not found
Antiretroviral dynamics determines HIV evolution and predicts therapy outcome
- Record: found
- Abstract: found
- Article: not found
Dolutegravir or Darunavir in Combination with Zidovudine or Tenofovir to Treat HIV

- Record: found
- Abstract: found
- Article: found