- Record: found
- Abstract: found
- Article: found
The emerging role and targetability of the TCA cycle in cancer metabolism
Read this article at
Abstract
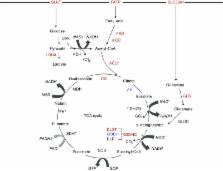
The tricarboxylic acid (TCA) cycle is a central route for oxidative phosphorylation in cells, and fulfills their bioenergetic, biosynthetic, and redox balance requirements. Despite early dogma that cancer cells bypass the TCA cycle and primarily utilize aerobic glycolysis, emerging evidence demonstrates that certain cancer cells, especially those with deregulated oncogene and tumor suppressor expression, rely heavily on the TCA cycle for energy production and macromolecule synthesis. As the field progresses, the importance of aberrant TCA cycle function in tumorigenesis and the potentials of applying small molecule inhibitors to perturb the enhanced cycle function for cancer treatment start to evolve. In this review, we summarize current knowledge about the fuels feeding the cycle, effects of oncogenes and tumor suppressors on fuel and cycle usage, common genetic alterations and deregulation of cycle enzymes, and potential therapeutic opportunities for targeting the TCA cycle in cancer cells. With the application of advanced technology and in vivo model organism studies, it is our hope that studies of this previously overlooked biochemical hub will provide fresh insights into cancer metabolism and tumorigenesis, subsequently revealing vulnerabilities for therapeutic interventions in various cancer types.
Related collections
Most cited references130
- Record: found
- Abstract: found
- Article: found
TIGAR, a p53-Inducible Regulator of Glycolysis and Apoptosis
- Record: found
- Abstract: found
- Article: not found
Reflecting on 25 years with MYC.
- Record: found
- Abstract: found
- Article: found