- Record: found
- Abstract: found
- Article: found
Changes in the expression of the type 2 diabetes-associated gene VPS13C in the β-cell are associated with glucose intolerance in humans and mice
Read this article at
Abstract
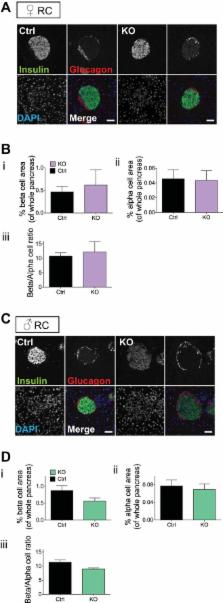
Single nucleotide polymorphisms (SNPs) close to the VPS13C, C2CD4A and C2CD4B genes on chromosome 15q are associated with impaired fasting glucose and increased risk of type 2 diabetes. eQTL analysis revealed an association between possession of risk (C) alleles at a previously implicated causal SNP, rs7163757, and lowered VPS13C and C2CD4A levels in islets from female ( n = 40, P < 0.041) but not from male subjects. Explored using promoter-reporter assays in β-cells and other cell lines, the risk variant at rs7163757 lowered enhancer activity. Mice deleted for Vps13c selectively in the β-cell were generated by crossing animals bearing a floxed allele at exon 1 to mice expressing Cre recombinase under Ins1 promoter control (Ins1Cre). Whereas Vps13c fl/fl:Ins1Cre (βVps13cKO) mice displayed normal weight gain compared with control littermates, deletion of Vps13c had little effect on glucose tolerance. Pancreatic histology revealed no significant change in β-cell mass in KO mice vs. controls, and glucose-stimulated insulin secretion from isolated islets was not altered in vitro between control and βVps13cKO mice. However, a tendency was observed in female null mice for lower insulin levels and β-cell function (HOMA-B) in vivo. Furthermore, glucose-stimulated increases in intracellular free Ca 2+ were significantly increased in islets from female KO mice, suggesting impaired Ca 2+ sensitivity of the secretory machinery. The present data thus provide evidence for a limited role for changes in VPS13C expression in conferring altered disease risk at this locus, particularly in females, and suggest that C2CD4A may also be involved.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
A genome-wide association study identifies novel risk loci for type 2 diabetes.
- Record: found
- Abstract: not found
- Article: not found
Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I.
- Record: found
- Abstract: found
- Article: not found