- Record: found
- Abstract: found
- Article: not found
Synthesis, structural insights, and biological screening of DNA targeted Ru(ii)(η 6- p-cymene) complexes containing bioactive amino-benzothiazole ligand scaffolds
Read this article at
Abstract
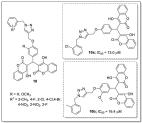
Two new drug candidates [Ru( p-cymene)(C 7H 4ClN 2S)Cl 2] and [Ru( p-cymene)(C 7H 5FN 2S)Cl 2] were synthesized and characterised. The in vitro cytotoxic activity of the complexes was assessed against five human cancer cell lines and anthelmintic activity was also investigated.
Abstract
Two new ruthenium-based drug candidates [Ru( p-cymene)(C 7H 4ClN 2S)Cl 2] (1) and [Ru( p-cymene)(C 7H 5FN 2S)Cl 2] (2) containing amino benzothiazole ligands were synthesized and comprehensively characterised by multiple spectroscopic and single crystal X-ray diffraction techniques. B3LYP computations were executed to evaluate the HOMO/LUMO energy gap. In vitro binding studies of 1 and 2 with ct-DNA were carried out by employing UV-vis, fluorescence, circular dichroic and cyclic voltammetric techniques, which revealed a stronger binding proclivity of 2 as compared to 1. Cleavage experiments with pBR322 DNA were carried out which revealed that both the complexes cleaved the DNA via an oxidative pathway. Protein interaction studies of both the complexes were accomplished by employing fluorescence studies, and it was observed that both the complexes could statically quench the intrinsic fluorescence indicating efficient binding interaction with bovine serum albumin. The in vitro cytotoxic activity of the complexes was assessed against five human cancer cell lines and further anthelmintic activity was also investigated. The results suggested that the complexes inhibited the glutathione transferase activity, increased the antioxidant potential and resulted in widespread tegmental damage, suggesting the interaction of 1 and 2 with β-tubulin. Furthermore, both the drug candidates demonstrated low cytotoxicity towards lymphocytes indicating their compatibility with mammalian cells.
Related collections
Most cited references64
- Record: found
- Abstract: not found
- Article: not found
Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase
- Record: found
- Abstract: found
- Article: found
A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications
- Record: found
- Abstract: found
- Article: not found
