- Record: found
- Abstract: found
- Article: found
Pluripotent stem cell‐derived retinal organoids for disease modeling and development of therapies
Read this article at
Abstract
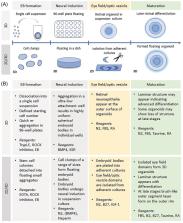
Retinal diseases constitute a genetically and phenotypically diverse group of clinical conditions leading to vision impairment or blindness with limited treatment options. Advances in reprogramming of somatic cells to induced pluripotent stem cells and generation of three‐dimensional organoids resembling the native retina offer promising tools to interrogate disease mechanisms and evaluate potential therapies for currently incurable retinal neurodegeneration. Next‐generation sequencing, single‐cell analysis, advanced electrophysiology, and high‐throughput screening approaches are expected to greatly expand the utility of stem cell‐derived retinal cells and organoids for developing personalized treatments. In this review, we discuss the current status and future potential of combining retinal organoids as human models with recent technologies to advance the development of gene, cell, and drug therapies for retinopathies.
Abstract
Related collections
Most cited references91
- Record: found
- Abstract: found
- Article: not found
Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis.
- Record: found
- Abstract: found
- Article: not found
