- Record: found
- Abstract: found
- Article: found
Output variability across animals and levels in a motor system
Read this article at
Abstract
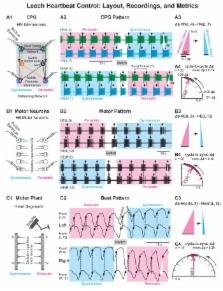
Rhythmic behaviors vary across individuals. We investigated the sources of this output variability across a motor system, from the central pattern generator (CPG) to the motor plant. In the bilaterally symmetric leech heartbeat system, the CPG orchestrates two coordinations in the bilateral hearts with different intersegmental phase relations (Δ ϕ) and periodic side-to-side switches. Population variability is large. We show that the system is precise within a coordination, that differences in repetitions of a coordination contribute little to population output variability, but that differences between bilaterally homologous cells may contribute to some of this variability. Nevertheless, much output variability is likely associated with genetic and life history differences among individuals. Variability of Δ ϕ were coordination-specific: similar at all levels in one, but significantly lower for the motor pattern than the CPG pattern in the other. Mechanisms that transform CPG output to motor neurons may limit output variability in the motor pattern.
eLife digest
Many of our everyday behaviors are rhythmic actions, such as walking, breathing and chewing. Networks of neurons called Central Pattern Generators, or CPGs, are in charge of rhythmic behaviors. CPGs send instructions to cells called motor neurons, which in turn tell muscles to contract in a particular sequence to produce rhythmic behaviors.
Rhythmic behaviors follow stereotyped patterns: we recognize walking when we see it. But they also vary between individuals: we can recognize the specific gait or ‘walk’ of a friend. Wenning et al. set out to discover where this variability in rhythmic behaviors comes from, using the leech heartbeat system as a model. Leeches have two hearts, or more precisely two heart tubes that run along the entire length of the body, one on either side. The two heart tubes beat with different patterns, but under the direction of the CPGs and motor neurons, they swap patterns with each other every few minutes. The CPG neurons that generate these rhythms, the motor neurons that respond, and the heart muscles themselves, i.e. each level of the system, can all be tracked in leeches.
Wenning et al. showed that within each leech, the activity of the CPG neurons, motor neurons and muscles associated with a heart tube varies little. Even when the activity of one of these levels varies less than another, for example between CPG and motor neurons, it is not necessarily reflected in the next level of the system. In some cases, however, variability is seen between opposite sides. Moreover, the rhythmic activity of CPG neurons, motor neurons, and muscle cells in one leech differs greatly from that of another. This likely reflects differences in the genes and life history of the animals.
Wenning et al. provide a roadmap for others to use in identifying sources of variability in rhythmic movements. Applying this approach to existing data sets could help tease apart variability in diverse rhythmic behaviors in a variety of animals.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
Variability, compensation and homeostasis in neuron and network function.
- Record: found
- Abstract: found
- Article: not found
Similar network activity from disparate circuit parameters.
- Record: found
- Abstract: found
- Article: not found