- Record: found
- Abstract: found
- Article: found
A Novel Method for Studying the Pharmacokinetics of [ 14C]Umeclidinium After Application to the Axilla or Palm of Healthy Male Subjects
Read this article at
Abstract
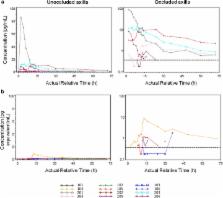
Umeclidinium (UMEC), a long‐acting muscarinic antagonist approved for chronic obstructive pulmonary disease (COPD), was investigated for primary hyperhidrosis as topical therapy. This study evaluated the pharmacokinetics, safety, and tolerability of a single dose of [ 14C]UMEC applied to either unoccluded axilla (UA), occluded axilla (OA), or occluded palm (OP) of healthy males. After 8 h the formulation was removed. [ 14C]UMEC plasma concentrations (Cp) were quantified by accelerator mass spectrometry. Occlusion increased systemic exposure by 3.8‐fold. Due to UMEC absorption‐limited pharmacokinetics, Cp data from the OA were combined with intravenous data from a phase I study. The data were described by a two‐compartment population model with sequential zero and first‐order absorption and linear elimination. Simulated systemic exposure following q.d. doses to axilla was similar to the exposure from the inhaled therapy, suggesting that systemic safety following dermal administration can be bridged to the inhaled program, and offering the potential for a reduced number of studies and/or subjects.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey.
- Record: found
- Abstract: not found
- Article: not found
Permeability of the skin.

- Record: found
- Abstract: found
- Article: found