- Record: found
- Abstract: found
- Article: found
Cancer risk at low doses of ionizing radiation: artificial neural networks inference from atomic bomb survivors
Read this article at
Abstract
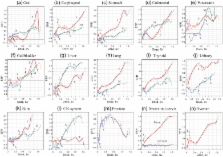
Cancer risk at low doses of ionizing radiation remains poorly defined because of ambiguity in the quantitative link to doses below 0.2 Sv in atomic bomb survivors in Hiroshima and Nagasaki arising from limitations in the statistical power and information available on overall radiation dose. To deal with these difficulties, a novel nonparametric statistics based on the ‘integrate-and-fire’ algorithm of artificial neural networks was developed and tested in cancer databases established by the Radiation Effects Research Foundation. The analysis revealed unique features at low doses that could not be accounted for by nominal exposure dose, including (i) the presence of a threshold that varied with organ, gender and age at exposure, and (ii) a small but significant bumping increase in cancer risk at low doses in Nagasaki that probably reflects internal exposure to 239Pu. The threshold was distinct from the canonical definition of zero effect in that it was manifested as negative excess relative risk, or suppression of background cancer rates. Such a unique tissue response at low doses of radiation exposure has been implicated in the context of the molecular basis of radiation–environment interplay in favor of recently emerging experimental evidence on DNA double-strand break repair pathway choice and its epigenetic memory by histone marking.
Related collections
Most cited references151
- Record: found
- Abstract: found
- Article: not found
DNA double-strand breaks: signaling, repair and the cancer connection.
- Record: found
- Abstract: found
- Article: not found
Regulation of DNA double-strand break repair pathway choice.
- Record: found
- Abstract: found
- Article: not found