- Record: found
- Abstract: found
- Article: found
Plitidepsin Has a Safe Cardiac Profile: A Comprehensive Analysis
Read this article at
Abstract
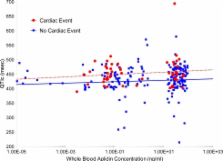
Plitidepsin is a cyclic depsipeptide of marine origin in clinical development in cancer patients. Previously, some depsipeptides have been linked to increased cardiac toxicity. Clinical databases were searched for cardiac adverse events (CAEs) that occurred in clinical trials with the single-agent plitidepsin. Demographic, clinical and pharmacological variables were explored by univariate and multivariate logistic regression analysis. Forty-six of 578 treated patients (8.0%) had at least one CAE (11 patients (1.9%) with plitidepsin-related CAEs), none with fatal outcome as a direct consequence. The more frequent CAEs were rhythm abnormalities ( n = 31; 5.4%), mostly atrial fibrillation/flutter ( n = 15; 2.6%). Of note, life-threatening ventricular arrhythmias did not occur. Myocardial injury events ( n = 17; 3.0%) included possible ischemic-related and non-ischemic events. Other events (miscellaneous, n = 6; 1.0%) were not related to plitidepsin. Significant associations were found with prostate or pancreas cancer primary diagnosis ( p = 0.0017), known baseline cardiac risk factors ( p = 0.0072), myalgia present at baseline ( p = 0.0140), hemoglobin levels lower than 10 g/dL ( p = 0.0208) and grade ≥2 hypokalemia ( p = 0.0095). Treatment-related variables (plitidepsin dose, number of cycles, schedule and/or total cumulative dose) were not associated. Electrocardiograms performed before and after plitidepsin administration ( n = 136) detected no relevant effect on QTc interval. None of the pharmacokinetic parameters analyzed had a significant impact on the probability of developing a CAE. In conclusion, the most frequent CAE type was atrial fibrillation/atrial flutter, although its frequency was not different to that reported in the age-matched healthy population, while other CAEs types were rare. No dose-cumulative pattern was observed, and no treatment-related variables were associated with CAEs. Relevant risk factors identified were related to the patient’s condition and/or to disease-related characteristics rather than to drug exposure. Therefore, the current analysis supports a safe cardiac risk profile for single-agent plitidepsin in cancer patients.
Related collections
Most cited references40
- Record: found
- Abstract: not found
- Article: not found
Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity.
- Record: found
- Abstract: found
- Article: not found
Aplidine, a new anticancer agent of marine origin, inhibits vascular endothelial growth factor (VEGF) secretion and blocks VEGF-VEGFR-1 (flt-1) autocrine loop in human leukemia cells MOLT-4.
- Record: found
- Abstract: found
- Article: not found