- Record: found
- Abstract: found
- Article: found
Painful and Painless Diabetic Neuropathies: What Is the Difference?
Read this article at
Abstract
Purpose of Review
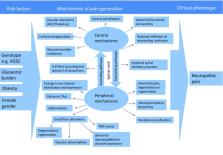
The prevalence of diabetes mellitus and its chronic complications are increasing to epidemic proportions. This will unfortunately result in massive increases in diabetic distal symmetrical polyneuropathy (DPN) and its troublesome sequelae, including disabling neuropathic pain (painful-DPN), which affects around 25% of patients with diabetes. Why these patients develop neuropathic pain, while others with a similar degree of neuropathy do not, is not clearly understood. This review will look at recent advances that may shed some light on the differences between painful and painless-DPN.
Recent Findings
Gender, clinical pain phenotyping, serum biomarkers, brain imaging, genetics, and skin biopsy findings have been reported to differentiate painful- from painless-DPN.
Summary
Painful-DPN seems to be associated with female gender and small fiber dysfunction. Moreover, recent brain imaging studies have found neuropathic pain signatures within the central nervous system; however, whether this is the cause or effect of the pain is yet to be determined. Further research is urgently required to develop our understanding of the pathogenesis of pain in DPN in order to develop new and effective mechanistic treatments for painful-DPN.
Related collections
Most cited references120
- Record: found
- Abstract: found
- Article: not found
New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain.
- Record: found
- Abstract: found
- Article: not found
The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study.
- Record: found
- Abstract: found
- Article: not found