- Record: found
- Abstract: found
- Article: found
MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction
Read this article at
Abstract
Background
Our study aim was to evaluate the therapeutic efficacy and mechanisms of miR-133-overexpressing mesenchymal stem cells (MSCs) on acute myocardial infarction.
Methods
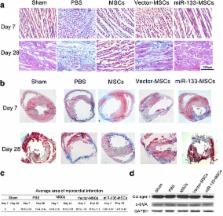
Rat MSCs were isolated and purified by whole bone marrow adherent culturing. After transfection with the agomir or antagomir of miR-133, MSCs were collected for assay of cell vitality, apoptosis, and cell cycle progression. At the same time, exosomes were isolated from the supernatant to analyze the paracrine miR-133. For in-vivo studies, constitutive activation of miR-133 in MSCs was achieved by lentivirus-mediated miR-133 overexpression. A rat myocardial infarction model was created by ligating the left anterior descending coronary artery, while control MSCs (vector-MSCs) or miR-133-overexpressed MSCs (miR-133-MSCs) were injected into the zone around the myocardial infarction. Subsequently, myocardial function was evaluated by echocardiography on days 7 and 28 post infarction. Finally the infarcted hearts were collected on days 7 and 28 for myocardial infarct size measurement and detection of snail 1 expression.
Results
Hypoxia-induced apoptosis of MSCs obviously reduced, along with enhanced expression of total poly ADP-ribose polymerase protein, after miR-133 agomir transfection, while the apoptosis rate increased in MSCs transfected with miR-133 antagomir. However, no change in cell viability and cell-cycle distribution was observed in control, miR-133-overexpressed, and miR-133-interfered MSCs. Importantly, rats transplanted with miR-133-MSCs displayed more improved cardiac function after acute myocardial infarction, compared with those that received vector-MSC injection. Further studies indicated that cardiac expression of snail 1 was significantly repressed by adjacent miR-133-overexpressing MSCs, and both the inflammatory level and the infarct size decreased in miR-133-MSC-injected rat hearts.
Related collections
Most cited references27
- Record: found
- Abstract: not found
- Article: not found
Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs.
- Record: found
- Abstract: found
- Article: not found
miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling.
- Record: found
- Abstract: found
- Article: not found