- Record: found
- Abstract: found
- Article: found
Do mHealth applications improve clinical outcomes of patients with cancer? A critical appraisal of the peer-reviewed literature
Read this article at
Abstract
Purpose
Patients undergoing systemic anti-cancer treatment experience distressing side effects, and these symptoms are often experienced outside the hospital setting. The impact of usage of cancer-related mobile health (mHealth) applications on patient-related outcomes requires investigation.
Methods
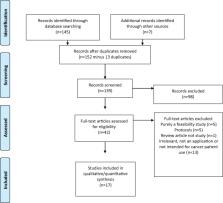
A critical appraisal of the literature was performed for the following question: ‘In patients with cancer have mHealth applications been compared with usual care to examine impact on commonly used clinical outcomes’. Literature searches were undertaken with the help of a research librarian and included Medline, Cochrane Collaboration, clinical trial databases and grey searches.
Results
Seventeen studies including between 12 and 2352 patients were identified and reviewed. Smartphone applications or internet portals collected data on symptoms or patient activity. Several studies showed statistically significant differences in patient-reported outcomes when symptom monitoring using mobile health application was compared to usual care. Change in mobility was the only outcome that was related directly to toxicity. Only limited data on mortality, cancer-related morbidity including complications of care, health-economic outcomes or long-term outcomes were reported.
Related collections
Most cited references36

- Record: found
- Abstract: found
- Article: found
Cancer Survivors’ Experience With Telehealth: A Systematic Review and Thematic Synthesis
- Record: found
- Abstract: not found
- Article: not found
Evaluating mobile phone applications for health behaviour change: A systematic review
- Record: found
- Abstract: found
- Article: found