- Record: found
- Abstract: found
- Article: found
Toll-Like Receptor 8 Ligands Activate a Vitamin D Mediated Autophagic Response that Inhibits Human Immunodeficiency Virus Type 1
Read this article at
Abstract
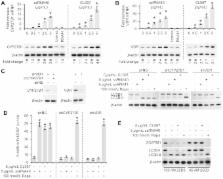
Toll-like receptors (TLR) are important in recognizing microbial pathogens and triggering host innate immune responses, including autophagy, and in the mediation of immune activation during human immunodeficiency virus type-1 (HIV) infection. We report here that TLR8 activation in human macrophages induces the expression of the human cathelicidin microbial peptide (CAMP), the vitamin D receptor (VDR) and cytochrome P450, family 27, subfamily B, polypeptide 1 (CYP27B1), which 1 α-hydroxylates the inactive form of vitamin D, 25-hydroxycholecalciferol, into its biologically active metabolite. Moreover, we demonstrate using RNA interference, chemical inhibitors and vitamin D deficient media that TLR8 agonists inhibit HIV through a vitamin D and CAMP dependent autophagic mechanism. These data support an important role for vitamin D in the control of HIV infection, and provide a biological explanation for the benefits of vitamin D. These findings also provide new insights into potential novel targets to prevent and treat HIV infection.
Author Summary
Cells use macroautophagy (autophagy - ‘ self-eating’, lysosome-dependent degradation and recycling of intracellular components in response to stress) as a mechanism to detect intracellular pathogens through pattern-recognition receptors such as Toll-like receptors (TLRs) that recognize signature molecules of pathogens that are essential for their survival. One such Toll-like receptor, TLR8, which is located in human macrophage endosomes, recognizes both imidazoquinoline compounds and uridine-rich single-stranded RNA such as human immunodeficiency virus type-1 (HIV) single-stranded RNA. In the present study we report that TLR8 activation in human macrophages induces the expression of the human cathelicidin microbial peptide (CAMP), the vitamin D receptor (VDR), and cytochrome P450, family 27, subfamily B, polypeptide 1 (CYP27B1), which 1 α-hydroxylates the inactive form of vitamin D, 25-hydroxycholecalciferol, into its biologically active metabolite. Moreover, we demonstrate that TLR8 activation induces autophagy in human macrophages through a vitamin D and CAMP dependent mechanism, and that the induction of autophagy by TLR8 agonists inhibits HIV. These data support an important role for vitamin D in the control of HIV infection, and provide a biological explanation for the benefits of vitamin D. These findings also provide new insights into potential novel targets to prevent and treat HIV infection.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Dissection of Autophagosome Formation Using Apg5-Deficient Mouse Embryonic Stem Cells
- Record: found
- Abstract: not found
- Article: not found
Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848.
- Record: found
- Abstract: found
- Article: not found