- Record: found
- Abstract: found
- Article: found
Inhibition of breast cancer cell proliferation and tumorigenesis by long non-coding RNA RPPH1 down-regulation of miR-122 expression
Read this article at
Abstract
Background
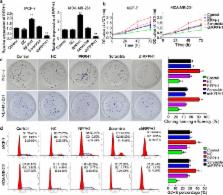
Recent studies showed that long non-coding RNA (lncRNA) plays an important role in many life activities. RPPH1 is one of the lncRNA genes that are expressed differently between breast cancer and normal tissues by the lncRNA gene chip. Our study was conducted to examine the regulation of lncRNA RPPH1 in breast cancer.
Methods
Two cell lines, MCF-7 and MDA-MB-231, were selected to be the research objects in this study; RPPH1 overexpression and knockdown models were established by transforming vectors. Real-time polymerase chain reaction, MTT assay, clone formation and cell flow cytometer assay were used to test the function of RPPH1. Dual-luciferase assay was used to detect a target relationship between RPPH1 and miR-122.
Results
RPPH1 overexpression promoted cell cycle and proliferation and increased colony formation. In the RPPH1 overexpression model, there was a target relationship between RPPH1 and miR-122, and some of the downstream genes of miR-122, including ADAM10, PKM2, NOD2 and IGF1R, were increased. Moreover, we found that lentivirus-mediated interference of lncRNA RPPH1 inhibited tumour growth in nude mice.
Related collections
Most cited references15

- Record: found
- Abstract: found
- Article: found
Long noncoding RNA associated-competing endogenous RNAs in gastric cancer

- Record: found
- Abstract: found
- Article: found
miR-31 and its host gene lncRNA LOC554202 are regulated by promoter hypermethylation in triple-negative breast cancer
- Record: found
- Abstract: found
- Article: not found