- Record: found
- Abstract: found
- Article: found
Rho-Associated Protein Kinase (ROCK) Promotes Proliferation and Migration of PC-3 and DU145 Prostate Cancer Cells by Targeting LIM Kinase 1 (LIMK1) and Matrix Metalloproteinase-2 (MMP-2)
Read this article at
Abstract
Background
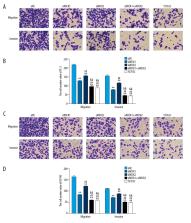
In the pathogenesis and progression of prostate cancer, cell proliferation and cell migration results in tumor invasion and metastasis that is associated with patient morbidity and mortality. Rho-associated protein kinase (ROCK) has previously been shown to be upregulated in prostate cancer, but its biological role remains poorly understood. This study aimed to investigate the role of ROCK in the proliferation and migration of PC-3 and DU145 prostate cancer cells and to identify the possible targets involved by knockdown of ROCK1 and ROCK2 RNA expression.
Material/Methods
An RNA interference (RNAi) assay was performed to silence the expression of ROCK1 and ROCK2 in the PC-3 and DU145 human prostate cancer cell lines. Cells were also treated with a specific ROCK inhibitor, Y27632. A cell counting kit-8 (CCK-8) assay was used to determine the proliferation rate of prostate cancer cells, and cell migration and invasion assays were performed. Western blot and polymerase chain reaction were used to measure protein and RNA expression levels.
Results
In PC-3 and DU145 prostate cancer cells, knockdown of ROCK1 and ROCK2 reduced cell migration and invasion. ROCK1 and ROCK2 regulated cell proliferation in PC-3 and DU145 prostate cancer cells. Protein levels of phosphorylated LIM kinase 1 (p-LIMK1) and matrix metalloproteinase-2 (MMP-2) were reduced in ROCK1 and ROCK2 siRNA transfected cells.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Cancer statistics, 2012.
- Record: found
- Abstract: found
- Article: not found
Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers.
- Record: found
- Abstract: found
- Article: not found