- Record: found
- Abstract: found
- Article: found
Evaluation of Disease Causality of Rare Ixodes ricinus-Borne Infections in Europe
Read this article at
Abstract
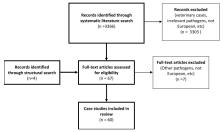
In Europe, Ixodes ricinus ticks transmit pathogens such as Borrelia burgdorferi sensu lato and tick-borne encephalitis virus (TBEV). In addition, there is evidence for transmission to humans from I. ricinus of Anaplasma phagocytophilum, Babesia divergens, Babesia microti, Babesia venatorum, Borrelia miyamotoi, Neoehrlichia mikurensis, Rickettsia helvetica and Rickettsia monacensis. However, whether infection with these potential tick-borne pathogens results in human disease has not been fully demonstrated for all of these tick-borne microorganisms. To evaluate the available evidence for a causative relation between infection and disease, the current study analyses European case reports published from 2008 to 2018, supplemented with information derived from epidemiological and experimental studies. The evidence for human disease causality in Europe found in this review appeared to be strongest for A. phagocytophilum and B. divergens. Nonetheless, some knowledge gaps still exist. Importantly, comprehensive evidence for pathogenicity is lacking for the remaining tick-borne microorganisms. Such evidence could be gathered best through prospective studies, for example, studies enrolling patients with a fever after a tick bite, the development of specific new serological tools, isolation of these microorganisms from ticks and patients and propagation in vitro, and through experimental studies.
Related collections
Most cited references158
- Record: found
- Abstract: not found
- Article: not found
Human babesiosis.

- Record: found
- Abstract: found
- Article: found
