- Record: found
- Abstract: found
- Article: not found
Challenges in the Diagnosis and Management of Growth Hormone Deficiency in India
Read this article at
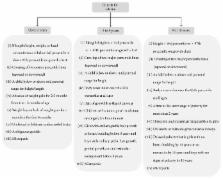
Abstract
In clinical practice, every year approximately 150,000 children are referred with short stature (SS) based on a cut-off of fifth percentile. The most important endocrine and treatable cause of SS is growth hormone deficiency (GHD). The lack of reliable data on the prevalence of GHD in India limits estimation of the magnitude of this problem. The diagnosis and treatment of GHD are hurdled with various challenges, restricting the availability of growth hormone (GH) therapy to only a very limited segment of the children in India. This review will firstly summarize the gaps and challenges in diagnosis and treatment of GHD based on literature analysis. Subsequently, it presents suggestions from the members at advisory board meetings to overcome these challenges. The advisory board suggested that early initiation of the therapy could better the chances of achieving final adult height within the normal range for the population. Education and awareness about growth disorders among parents, regular training for physicians, and more emphasis on using the Indian growth charts for growth monitoring would help improve the diagnosis and treatment of children with GHD. Availability of an easy-to-use therapy delivery system could also be beneficial in improving adherence and achieving satisfactory outcomes.
Related collections
Most cited references73
- Record: found
- Abstract: found
- Article: not found
Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop.
- Record: found
- Abstract: not found
- Article: not found
Standards for children's height at ages 2-9 years allowing for heights of parents.
- Record: found
- Abstract: found
- Article: not found
