- Record: found
- Abstract: found
- Article: found
Effect of Duration and Intermittency of Rifampin on Tuberculosis Treatment Outcomes: A Systematic Review and Meta-Analysis
Read this article at
Abstract
In a systematic review of randomized controlled trials on tuberculosis treatment, Dick Menzies and colleagues find shorter courses of rifampin to be associated with poorer treatment outcomes.
Abstract
Background
Treatment regimens for active tuberculosis (TB) that are intermittent, or use rifampin during only the initial phase, offer practical advantages, but their efficacy has been questioned. We conducted a systematic review of treatment regimens for active TB, to assess the effect of duration and intermittency of rifampin use on TB treatment outcomes.
Methods and Findings
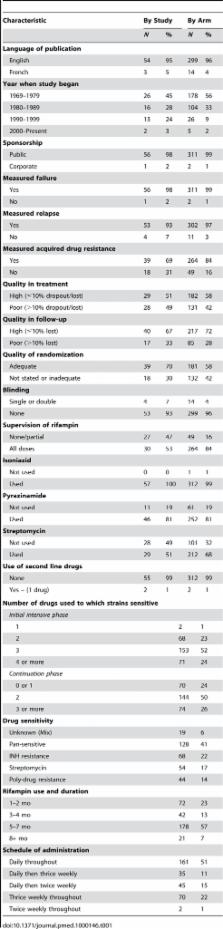
PubMed, Embase, and the Cochrane CENTRAL database for clinical trials were searched for randomized controlled trials, published in English, French, or Spanish, between 1965 and June 2008. Selected studies utilized standardized treatment with rifampin-containing regimens. Studies reported bacteriologically confirmed failure and/or relapse in previously untreated patients with bacteriologically confirmed pulmonary TB. Pooled cumulative incidences of treatment outcomes and association with risk factors were computed with stratified random effects meta-analyses. Meta-regression was performed using a negative binomial regression model. A total of 57 trials with 312 arms and 21,472 participants were included in the analysis. Regimens utilizing rifampin only for the first 1–2 mo had significantly higher rates of failure, relapse, and acquired drug resistance, as compared to regimens that used rifampin for 6 mo. This was particularly evident when there was initial drug resistance to isoniazid, streptomycin, or both. On the other hand, there was little evidence of difference in failure or relapse with daily or intermittent schedules of treatment administration, although there was insufficient published evidence of the efficacy of twice-weekly rifampin administration throughout therapy.
Conclusions
TB treatment outcomes were significantly worse with shorter duration of rifampin, or with initial drug resistance to isoniazid and/or streptomycin. Treatment outcomes were similar with all intermittent schedules evaluated, but there is insufficient evidence to support administration of treatment twice weekly throughout therapy.
Editors' Summary
Background
Tuberculosis—a contagious infection, usually of the lungs—kills nearly two million people annually. It is caused by Mycobacterium tuberculosis, bacteria that are spread in airborne droplets when people with tuberculosis cough. Most people infected with M. tuberculosis do not become ill—their immune system contains the infection. However, the bacteria remain dormant within the body and can cause tuberculosis years later if immunity declines because of, for example, infection with HIV (the virus that causes AIDS). The symptoms of tuberculosis include a persistent cough, weight loss and night sweats. The disease can usually be cured by taking several powerful antibiotics regularly for several months although drug-resistant tuberculosis is increasingly widespread. The standardized drug regimen recommended by the World Health Organization (WHO) for previously uninfected patients consists of an initial treatment phase, in which rifampin, isoniazid, ethambutol, and pyrazinamide are taken daily or thrice weekly for 2 months, and a continuation phase, in which two antibiotics are taken for a further 4–6 months.
Why Was This Study Done?
Resistance to rifampicin, which can develop if this drug is not taken regularly, is associated with poor treatment outcomes, particularly in patients infected with isoniazid-resistant M. tuberculosis. WHO recommends, therefore, that health-care workers watch patients take all their doses of rifampicin (“directly observed therapy”). Treatment regimens for tuberculosis that use rifampicin only during the initial phase and/or give rifampicin several times a week (intermittently) rather than daily would make direct observation of treatment much easier but are such regimens effective? In this study (which, together with two similar studies, was commissioned by WHO to provide the evidence needed for a revision of their treatment guidelines), the researchers undertook a systematic review (a search using specific criteria to identify relevant research studies, which are then appraised and analyzed according to an explicit protocol) and a meta-analysis (a statistical approach that pools the results of several studies) of published trials of various rifampicin-containing regimens for the treatment of tuberculosis in previously untreated patients.
What Did the Researchers Do and Find?
The researchers identified 57 randomized controlled trials (studies in which groups of patients are randomly assigned to different interventions) that reported the treatment failure and/or relapse rates (determined by seeing whether M. tuberculosis could be grown from sputum brought up from the lungs by coughing, so-called bacteriologically confirmed tuberculosis) associated with various rifampicin-containing treatment regimens. In their statistical analysis of the results of these trials (which involved more than 21,000 previously untreated patients), the researchers found that regimens that used rifampicin during only the first 1–2 months of treatment had higher rates of failure, relapse, and acquired drug resistance than regimens that used rifampicin for 6 months. Indeed, relapse rates decreased with the duration of rifampicin treatment up to 8 months of treatment. Furthermore, outcomes were particularly bad with regimens that included rifampicin during only the first 1–2 months of treatment if there was initial resistance to isoniazid and/or streptomycin (another antibiotic). Outcomes were similar, however, in regimens in which rifampicin was given daily throughout treatment, daily during the initial phase then twice or thrice weekly, or thrice weekly throughout treatment; insufficient evidence was available to evaluate the efficacy of regimens in which rifampicin was given twice weekly throughout treatment.
What Do These Findings Mean?
These findings suggest that tuberculosis treatment regimens for previously untreated patients who use rifampicin during only the first two months of treatment should be phased out and replaced by regimens that use rifampicin for 6 months, particularly in settings where there is likely to be resistance to isoniazid and/or streptomycin. This recommendation will be made in the planned 2009 revision of the WHO tuberculosis treatment guidelines. In addition, these findings suggest that giving rifampicin thrice weekly is as effective as giving it daily during the initial phase or throughout treatment. Importantly, these findings also indicate that more trials are needed to investigate other dosing schedules, to determine the optimal duration of treatment, and to determine the best way to manage patients infected with isoniazid-resistant bacteria. Finally, since very few of the trials identified in the systematic review included HIV-positive participants, trials designed to test drug regimens in people infected with both HIV and M. tuberculosis are urgently needed to reduce global deaths from tuberculosis.
Additional Information
Please access these Web sites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.1000146.
-
The results of another WHO-commissioned study into the treatment of tuberculosis are presented in a separate PLoS Medicine Research Article by Menzies et al. (Menzies D, Benedetti A, Paydar A, Royce S, Pai M, et al. (2009) Standardized Treatment of Active Tuberculosis in Patients with Previous Treatment and/or with Mono-resistance to Isoniazid: A Systematic Review and Meta-analysis. PLoS Med 6(9): e1000150. doi:10.1371/journal.pmed.1000150)
-
The US National Institute of Allergy and Infectious Diseases provides information on all aspects of tuberculosis
-
The US Centers for Disease Control and Prevention provide several facts sheets and other information resources about tuberculosis
-
The American Thoracic Society, US Centers for Disease Control and Prevention, and Infectious Diseases Society of America have published guidelines on TB treatment
-
The 2003 (2004 revision) WHO guidelines for national programs for the treatment of tuberculosis are available; WHO also provides information on efforts to reduce the global burden of tuberculosis (in several languages) and its 2009 annual report on global control of tuberculosis describes the current situation (key points are available in several languages)
-
The WHO publishes guidelines on TB treatment
Related collections
Most cited references71
- Record: found
- Abstract: not found
- Article: not found
American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis.
- Record: found
- Abstract: found
- Article: not found
Explaining heterogeneity in meta-analysis: a comparison of methods.
- Record: found
- Abstract: not found
- Article: not found
