- Record: found
- Abstract: found
- Article: found
Combining multi‐omics analysis to identify host‐targeted targets for the control of Brucella infection
Read this article at
Abstract
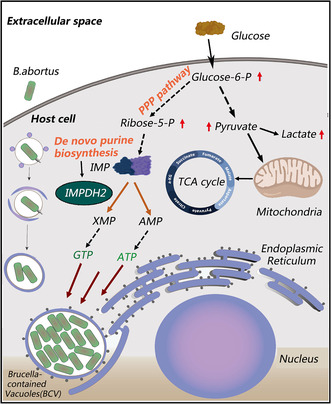
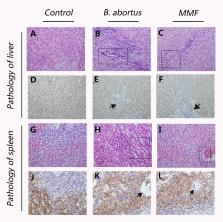
Human infections caused by Brucella (called brucellosis) are among the most common zoonoses worldwide with an estimated 500,000 cases each year. Since chronic Brucella infections are extremely difficult to treat, there is an urgent need for more effective therapeutics. As a facultative intracellular bacterium, Brucella is strictly parasitic in the host cell. Here, we performed proteomic and transcriptomic and metabolomic analyses on Brucella infected patients, mice and cells that provided an extensive “map” of physiological changes in brucellosis patients and characterized the metabolic pathways essential to the response to infection, as well as the associated cellular response and molecular mechanisms. This is the first report utilizing multi‐omics analysis to investigate the global response of proteins and metabolites associated with Brucella infection, and the data can provide a comprehensive insight to understand the mechanism of Brucella infection. We demonstrated that Brucella increased nucleotide synthesis in the host, consistent with increased biomass requirement. We also identified IMPDH2, a key regulatory complex that controls nucleotide synthesis during Brucella infection. Pharmacological targeting of IMPDH2, the rate‐limiting enzyme in guanine nucleotide biosynthesis, efficiently inhibits B. abortus growth both in vitro and in vivo. Through screening a library of natural products, we identified oxymatrine, an alkaloid obtained primarily from Sophora roots, is a novel and selective IMPDH2 inhibitor. In further in vitro bacterial inhibition assays, oxymatrine effectively inhibited the growth of B. abortus, which was impaired by exogenous supplementation of guanosine, a salvage pathway of purine nucleotides. This moderately potent, structurally novel compound may provide clues for further design and development of efficient IMPDH2 inhibitors and also demonstrates the potential of natural compounds from plants against Brucella.
Abstract
The infection caused by Brucella leads to a change in the host's metabolism, specifically to aerobic glycolysis. In this study, the researchers focused on investigating the role of inosine‐5'‐monophosphate dehydrogenase (IMPDH2), a crucial regulatory complex involved in controlling glucose and nucleotide metabolism during Brucella infection. The researchers determined that pharmacologically targeting IMPDH2 can effectively inhibit the growth of Brucella both in vitro and in vivo.
Related collections
Most cited references36

- Record: found
- Abstract: found
- Article: found
In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo
- Record: found
- Abstract: found
- Article: not found
PPARγ-mediated increase in glucose availability sustains chronic Brucella abortus infection in alternatively activated macrophages.

- Record: found
- Abstract: found
- Article: found