- Record: found
- Abstract: found
- Article: found
Short-term fasting in glioma patients: analysis of diet diaries and metabolic parameters of the ERGO2 trial
Read this article at
Abstract
Purpose
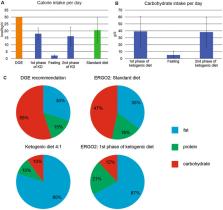
The prospective, randomized ERGO2 trial investigated the effect of calorie-restricted ketogenic diet and intermittent fasting (KD-IF) on re-irradiation for recurrent brain tumors. The study did not meet its primary endpoint of improved progression-free survival in comparison to standard diet (SD). We here report the results of the quality of life/neurocognition and a detailed analysis of the diet diaries.
Methods
50 patients were randomized 1:1 to re-irradiation combined with either SD or KD-IF. The KD-IF schedule included 3 days of ketogenic diet (KD: 21–23 kcal/kg/d, carbohydrate intake limited to 50 g/d), followed by 3 days of fasting and again 3 days of KD. Follow-up included examination of cognition, quality of life and serum samples.
Results
The 20 patients who completed KD-IF met the prespecified goals for calorie and carbohydrate restriction. Substantial decreases in leptin and insulin and an increase in uric acid were observed. The SD group, of note, had a lower calorie intake than expected (21 kcal/kg/d instead of 30 kcal/kg/d). Neither quality of life nor cognition were affected by the diet. Low glucose emerged as a significant prognostic parameter in a best responder analysis.
Conclusion
The strict caloric goals of the ERGO2 trial were tolerated well by patients with recurrent brain cancer. The short diet schedule led to significant metabolic changes with low glucose emerging as a candidate marker of better prognosis. The unexpected lower calorie intake of the control group complicates the interpretation of the results.
Clinicaltrials.gov number: NCT01754350; Registration: 21.12.2012.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: found
Hallmarks of Cancer: The Next Generation
- Record: found
- Abstract: found
- Article: not found
Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor.
- Record: found
- Abstract: found
- Article: not found