- Record: found
- Abstract: found
- Article: not found
miR-17–92 cluster: ups and downs in cancer and aging
Read this article at
Abstract
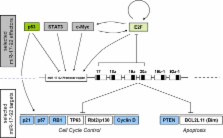
The miR-17–92 cluster encoding 6 single mature miRNAs was identified a couple of years ago to contain the first oncogenic miRNAs. Now, one of these 6 miRNAs, miR-19 has been identified as the key responsible for this oncogenic activity. This in turn reduces PTEN levels and in consequence activates the AKT/mTOR pathway that is also prominently involved in modulation of organismal life spans. In contrast, miR-19 and other members of the miR-17–92 cluster are found to be commonly downregulated in several human replicative and organismal aging models. Taken together, these findings suggest that miR-19 and the other members of the miR-17–92 cluster might be important regulators on the cross-roads between aging and cancer. Therefore, we here briefly summarize how this cluster is transcriptionally regulated, which target mRNAs have been confirmed so far and how this might be linked to modulation of organismal life-spans.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Chemokine signaling via the CXCR2 receptor reinforces senescence.
- Record: found
- Abstract: found
- Article: not found
