- Record: found
- Abstract: found
- Article: found
Pressure-induced planar N 6 rings in potassium azide
Read this article at
Abstract
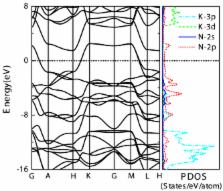
The first-principles method and the evolutionary algorithm are used to identify stable
high pressure phases of potassium azide (KN
3). It has been verified that the stable phase with space group
I4/
mcm below 22 GPa, which is consistent with the experimental result, will transform into
the
C2/
m phase with pressure increasing. These two phases are insulator with
 anions. A metallic phase with
P6/
mmm symmetry is preferred above 40 GPa, and the N atoms in this structure form six-membered
rings which are important for understanding the pressure effect on
anions. A metallic phase with
P6/
mmm symmetry is preferred above 40 GPa, and the N atoms in this structure form six-membered
rings which are important for understanding the pressure effect on
 anions and phase transitions of KN
3. Above the studied pressure (100 GPa), a polymerization of N
6 rings may be obtained as the result of the increasing compactness.
anions and phase transitions of KN
3. Above the studied pressure (100 GPa), a polymerization of N
6 rings may be obtained as the result of the increasing compactness.
Related collections
Most cited references14
- Record: found
- Abstract: not found
- Article: not found
Generalized Gradient Approximation Made Simple.
- Record: found
- Abstract: found
- Article: not found