- Record: found
- Abstract: found
- Article: found
SOX2 Reprograms Resident Astrocytes into Neural Progenitors in the Adult Brain
Read this article at
Summary
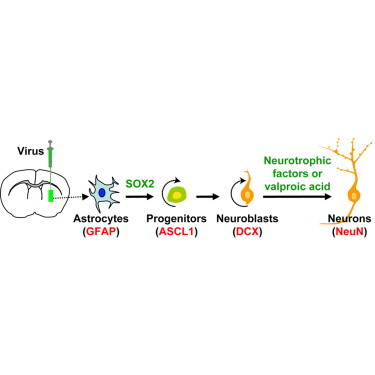
Glial cells can be in vivo reprogrammed into functional neurons in the adult CNS; however, the process by which this reprogramming occurs is unclear. Here, we show that a distinct cellular sequence is involved in SOX2-driven in situ conversion of adult astrocytes to neurons. This includes ASCL1 + neural progenitors and DCX + adult neuroblasts (iANBs) as intermediates. Importantly, ASCL1 is required, but not sufficient, for the robust generation of iANBs in the adult striatum. These progenitor-derived iANBs predominantly give rise to calretinin + interneurons when supplied with neurotrophic factors or the small-molecule valproic acid. Patch-clamp recordings from the induced neurons reveal subtype heterogeneity, though all are functionally mature, fire repetitive action potentials, and receive synaptic inputs. Together, these results show that SOX2-mediated in vivo reprogramming of astrocytes to neurons passes through proliferative intermediate progenitors, which may be exploited for regenerative medicine.
Graphical Abstract
Highlights
Abstract
In this article, Zhang and colleagues reveal that SOX2-mediated in vivo reprogramming of adult astrocytes transits through ASCL1-positive neural progenitors. These progenitors further generate DCX-positive neuroblasts and functionally mature neurons in the adult mouse striatum. This stepwise and expandable in vivo reprogramming process may be exploited for neural regeneration by using resident glial cells.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Direct conversion of fibroblasts to functional neurons by defined factors
- Record: found
- Abstract: found
- Article: not found
In vivo reprogramming of adult pancreatic exocrine cells to beta-cells.
- Record: found
- Abstract: found
- Article: not found