- Record: found
- Abstract: found
- Article: found
Genetically modified human placenta-derived mesenchymal stem cells with FGF-2 and PDGF-BB enhance neovascularization in a model of hindlimb ischemia
Read this article at
Abstract
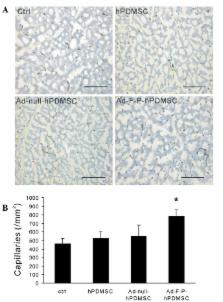
Ischemic diseases represent a challenging worldwide health burden. The current study investigated the therapeutic potential of genetically modified human placenta-derived mesenchymal stem cells (hPDMSCs) with basic fibroblast growth factor (FGF2) and platelet-derived growth factor-BB (PDGF-BB) genes in hindlimb ischemia. Mesenchymal stem cells obtained from human term placenta were transfected ex vivo with adenoviral bicistronic vectors carrying the FGF2 and PDGF-BB genes (Ad-F-P). Unilateral hindlimb ischemia was surgically induced by excision of the right femoral artery in New Zealand White rabbits. Ad-F-P genetically modified hPDMSCs, Ad-null (control vector)-modified hPDMSCs, unmodified hPDMSCs or media were intramuscularly implanted into the ischemic limbs 7 days subsequent to the induction of ischemia. Four weeks after cell therapy, angiographic analysis revealed significantly increased collateral vessel formation in the Ad-F-P-hPDMSC group compared with the control group. Histological examination revealed markedly increased capillary and arteriole density in the Ad-F-P-hPDMSC group. The xenografted hPDMSCs survived in the ischemic tissue for at least 4 weeks subsequent to cell therapy. The current study demonstrated that the combination of hPDMSC therapy with FGF2 and PDGF-BB gene therapy effectively induced collateral vessel formation and angiogenesis, suggesting a novel strategy for therapeutic angiogenesis.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells.
- Record: found
- Abstract: found
- Article: not found
Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms.
- Record: found
- Abstract: found
- Article: not found