- Record: found
- Abstract: found
- Article: found
Oral and intranasal vaccines against SARS‐CoV‐2: Current progress, prospects, advantages, and challenges
Read this article at
Abstract
Background
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has caused a deadly pandemic in the 21st century, resulting in many deaths, economic loss, and international immobility. Vaccination represents the only mechanism to defeat this virus. Several intramuscular vaccines have been approved and are currently used worldwide.
Main body
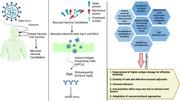
However, global mass vaccination has not been achieved owing to several limitations, including the need for expertise to administer the injection‐based vaccine, improper distribution of the vaccine, and lack of cold chain facilities, particularly in resource‐poor, low‐income countries. Mucosal vaccines are typically administered either orally or nasally, and several studies have shown promising results for developing these vaccines against SARS‐CoV‐2 that might serve as viable alternatives to current vaccines. SARS‐CoV‐2 invades the human body via oral and nasal mucosal surfaces; thus, an oral or nasal vaccine can trigger the immune system to inhibit the virus at the mucosal level, preventing further transmission via a strong mucosal and systematic immune response. Although several approaches toward developing a mucosal vaccine are currently being tested, additional attention is required.
Abstract
Mucosal vaccines are typically administered either orally or nasally, and several studies have shown promising results for developing these vaccines against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) that might serve as a viable alternative to current vaccines. SARS‐CoV‐2 invades the human body via oral and nasal mucosal surfaces; thus, an oral or nasal vaccine can trigger the immune system to inhibit the virus at the mucosal level, preventing further transmission via a strong mucosal and systematic immune response.
Related collections
Most cited references103
- Record: found
- Abstract: found
- Article: not found
SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor
- Record: found
- Abstract: found
- Article: not found
