- Record: found
- Abstract: found
- Article: found
Vesicular nanocarrier based treatment of skin fungal infections: Potential and emerging trends in nanoscale pharmacotherapy
Read this article at
Abstract
Occurrence of skin fungal infections is increasing nowadays and their presence is more prominent in patients suffering from immunocompromised diseases like AIDS. Skin fungal infections are a major cause of visits by patients to dermatology clinics. Although, a large number of antifungal agents are available for treatment of skin fungal infections, but, their toxic profile and physicochemical characteristics reduce therapeutic outcome. When these antifungal agents are delivered topically using conventional formulations like creams and gels, they may cause various side effects like redness, burning, and swelling at the site of application. Therefore, various vesicular formulations (phospholipid based or non phospholipid based) have been explored by pharmaceutical scientists to treat skin fungal infections topically. Vesicular formulation explored for the purpose are liposomes, ethosomes, transfersomes, transethosomes, niosomes, spanlastics, oleic acid vesicles, and nanoparticles. These formulations show various advantages like bioavailability enhancement of bioactives, high skin permeation power, no side effects at application site, dosing frequency reduction, and sustained drug release. Therefore, in the present article, we have discussed about the utility of various vesicular nanocarrier systems to treat skin fungal infections.
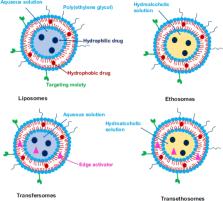
Graphical abstract
Related collections
Most cited references87

- Record: found
- Abstract: found
- Article: found
Liposomes as nanomedical devices
- Record: found
- Abstract: found
- Article: not found
Nanoparticles and microparticles for skin drug delivery.
- Record: found
- Abstract: found
- Article: not found
