- Record: found
- Abstract: found
- Article: found
Titanium Dioxide Nanoparticles in Food and Personal Care Products—What Do We Know about Their Safety?
Read this article at
Abstract
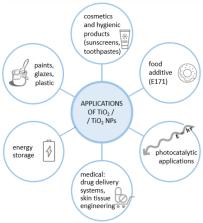
Titanium dioxide (TiO 2) is a material of diverse applications commonly used as a food additive or cosmetic ingredient. Its prevalence in products of everyday use, especially in nanosize, raises concerns about safety. Current findings on the safety of titanium dioxide nanoparticles (TiO 2 NPs) used as a food additive or a sunscreen compound are reviewed and systematized in this publication. Although some studies state that TiO 2 NPs are not harmful to humans through ingestion or via dermal exposure, there is a considerable number of data that demonstrated their toxic effects in animal models. The final agreement on the safety of this nanomaterial has not yet been reached among researchers. There is also a lack of official, standardized guidelines for thorough characterization of TiO 2 NPs in food and cosmetic products, provided by international authorities. Recent advances in the application of ‘green-synthesized’ TiO 2 NPs, as well as comparative studies of the properties of ‘biogenic’ and ‘traditional’ nanoparticles, are presented. To conclude, perspectives and directions for further studies on the toxicity of TiO 2 NPs are proposed.
Related collections
Most cited references124

- Record: found
- Abstract: found
- Article: found
‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation
- Record: found
- Abstract: found
- Article: not found
Titanium dioxide nanoparticles in food and personal care products.

- Record: found
- Abstract: found
- Article: found