- Record: found
- Abstract: found
- Article: found
Syndecan-4 Regulates Muscle Differentiation and Is Internalized from the Plasma Membrane during Myogenesis
Read this article at
Abstract
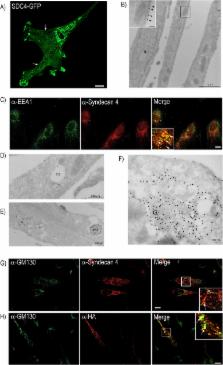
The cell surface proteoglycan syndecan-4 has been reported to be crucial for muscle differentiation, but the molecular mechanisms still remain to be fully understood. During in vitro differentiation of bovine muscle cells immunocytochemical analyses showed strong labelling of syndecan-4 intracellularly, in close proximity with Golgi structures, in membranes of intracellular vesicles and finally, in the nuclear area including the nuclear envelope. Chase experiments showed that syndecan-4 was internalized from the plasma membrane during this process. Furthermore, when syndecan-4 was knocked down by siRNA more myotubes were formed, and the expression of myogenic transcription factors, β1-integrin and actin was influenced. However, when bovine muscle cells were treated with a cell-penetrating peptide containing the cytoplasmic region of syndecan-4, myoblast fusion and thus myotube formation was blocked, both in normal cells and in syndecan-4 knock down cells. Altogether this suggests that the cytoplasmic domain of syndecan-4 is important in regulation of myogenesis. The internalization of syndecan-4 from the plasma membrane during muscle differentiation and the nuclear localization of syndecan-4 in differentiated muscle cells may be part of this regulation, and is a novel aspect of syndecan biology which merits further studies.
Related collections
Most cited references39

- Record: found
- Abstract: found
- Article: found
MIQE précis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments
- Record: found
- Abstract: found
- Article: not found