- Record: found
- Abstract: found
- Article: found
Oral submucous fibrosis stimulates invasion and epithelial‐mesenchymal transition in oral squamous cell carcinoma by activating MMP‐2 and IGF‐IR
Read this article at
Abstract
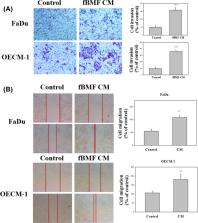
Oral submucous fibrosis (OSF) involves a high risk of malignant transformation and has been implicated in oral cancer. Limited studies have been conducted on the role of OSF in relation to the invasive capabilities and epithelial‐mesenchymal transition (EMT) in oral cancer. Herein, we investigated the effects of OSF on the microenvironment of human oral cancer cells. The results showed that the conditioned medium (CM) of fibrotic buccal mucosal fibroblasts (fBMFs) strongly induced the invasion of oral cancer cells and increased the activities of matrix metalloproteinase‐2. OSF significantly induced the EMT in oral cancer cells and downregulated epithelial markers, such as E‐cadherin, but significantly elevated vimentin, fibronectin, N‐cadherin, RhoA, Rac‐1 and FAK. Insulin‐like growth factor‐1 (IGF‐1) was elevated in OSF. The protein levels of the IGF‐1R were upregulated specifically in fBMF CM treatment for oral cancer cells, and the IGFR gene was confirmed by The Cancer Genome Atlas patient transcriptome data. The Kaplan‐Meier curve analysis revealed that patients with oral squamous cell carcinoma and high IGFR expression levels had poorer 5‐year survival than those with low IGFR expression ( p = 0.004). The fBMF‐stimulated EMT cell model may recapture some of the molecular changes during EMT progression in clinical patients with oral cancer.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
The biology and function of fibroblasts in cancer.
- Record: found
- Abstract: found
- Article: not found
Every step of the way: integrins in cancer progression and metastasis
- Record: found
- Abstract: not found
- Article: not found