- Record: found
- Abstract: found
- Article: found
EGFR pathway biomarkers in erlotinib-treated patients with advanced pancreatic cancer: translational results from the randomised, crossover phase 3 trial AIO-PK0104
Read this article at
Abstract
Background:
We aimed to identify molecular epidermal growth factor receptor (EGFR) tissue biomarkers in pancreatic cancer (PC) patients treated with the anti-EGFR agent erlotinib within the phase 3 randomised AIO-PK0104 study.
Methods:
AIO-PK0104 was a multicenter trial comparing gemcitabine/erlotinib followed by capecitabine with capecitabine/erlotinib followed by gemcitabine in advanced PC; primary study end point was the time-to-treatment failure after first- and second-line therapy (TTF2). Translational analyses were performed for KRAS exon 2 mutations, EGFR expression, PTEN expression, the EGFR intron 1 and exon 13 R497K polymorphism (PM). Biomarker data were correlated with TTF, overall survival (OS) and skin rash.
Results:
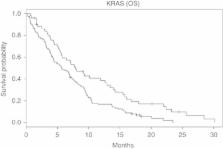
Archival tumour tissue was available from 208 (74%) of the randomised patients. The KRAS mutations were found in 70% (121 out of 173) of patients and exclusively occurred in codon 12. The EGFR overexpression was detected in 89 out of 181 patients (49%) by immunohistochemistry (IHC), and 77 out of 166 patients (46%) had an EGFR gene amplification by fluorescence in-situ hybridisation (FISH); 30 out of 171 patients (18%) had a loss of PTEN expression, which was associated with an inferior TTF1 (first-line therapy; HR 0.61, P=0.02) and TTF2 (HR 0.66, P=0.04). The KRAS wild-type status was associated with improved OS (HR 1.68, P=0.005); no significant OS correlation was found for EGFR–IHC (HR 0.96), EGFR–FISH (HR 1.22), PTEN–IHC (HR 0.77), intron 1 (HR 0.91) or exon 13 R497K PM (HR 0.83). None of the six biomarkers correlated with the occurrence of skin rash.