- Record: found
- Abstract: found
- Article: found
Automated segmentation by pixel classification of retinal layers in ophthalmic OCT images
Read this article at
Abstract
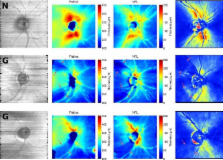
Current OCT devices provide three-dimensional (3D) in-vivo images of the human retina. The resulting very large data sets are difficult to manually assess. Automated segmentation is required to automatically process the data and produce images that are clinically useful and easy to interpret. In this paper, we present a method to segment the retinal layers in these images. Instead of using complex heuristics to define each layer, simple features are defined and machine learning classifiers are trained based on manually labeled examples. When applied to new data, these classifiers produce labels for every pixel. After regularization of the 3D labeled volume to produce a surface, this results in consistent, three-dimensionally segmented layers that match known retinal morphology. Six labels were defined, corresponding to the following layers: Vitreous, retinal nerve fiber layer (RNFL), ganglion cell layer & inner plexiform layer, inner nuclear layer & outer plexiform layer, photoreceptors & retinal pigment epithelium and choroid. For both normal and glaucomatous eyes that were imaged with a Spectralis (Heidelberg Engineering) OCT system, the five resulting interfaces were compared between automatic and manual segmentation. RMS errors for the top and bottom of the retina were between 4 and 6 μm, while the errors for intra-retinal interfaces were between 6 and 15 μm. The resulting total retinal thickness maps corresponded with known retinal morphology. RNFL thickness maps were compared to GDx (Carl Zeiss Meditec) thickness maps. Both maps were mostly consistent but local defects were better visualized in OCT-derived thickness maps.
Related collections
Most cited references16
- Record: found
- Abstract: not found
- Article: not found
Support-vector networks

- Record: found
- Abstract: found
- Article: found
Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation
- Record: found
- Abstract: found
- Article: not found