- Record: found
- Abstract: found
- Article: found
Clinical and Serological Findings of Madariaga and Venezuelan Equine Encephalitis Viral Infections: A Follow-up Study 5 Years After an Outbreak in Panama

Read this article at
Abstract
Background
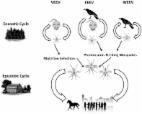
Human cases of Madariaga virus (MADV) infection were first detected during an outbreak in 2010 in eastern Panama, where Venezuelan equine encephalitis virus (VEEV) also circulates. Little is known about the long-term consequences of either alphavirus infection.
Methods
A follow-up study of the 2010 outbreak was undertaken in 2015. An additional survey was carried out 2 weeks after a separate 2017 alphavirus outbreak in a neighboring population in eastern Panama. Serological studies and statistical analyses were undertaken in both populations.
Results
Among the originally alphavirus-seronegative participants (n = 35 of 65), seroconversion was observed at a rate of 14.3% (95% CI, 4.8%–30.3%) for MADV and 8.6% (95% CI, 1.8%–23.1%) for VEEV over 5 years. Among the originally MADV-seropositive participants (n = 14 of 65), VEEV seroconversion occurred in 35.7% (95% CI, 12.8%–64.9%). In the VEEV-seropositive participants (n = 16 of 65), MADV seroconversion occurred in 6.3% (95% CI, 0.2%–30.2%). MADV seroreversion was observed in 14.3% (95% CI, 1.8%–42.8%) of those who were originally seropositive in 2010. VEEV seroconversion in the baseline MADV-seropositive participants was significantly higher than in alphavirus-negative participants. In the population sampled in 2017, MADV and VEEV seroprevalence was 13.2% and 16.8%, respectively. Memory loss, insomnia, irritability, and seizures were reported significantly more frequently in alphavirus-seropositive participants than in seronegative participants.
Conclusions
High rates of seroconversion to MADV and VEEV over 5 years suggest frequent circulation of both viruses in Panama. Enhanced susceptibility to VEEV infection may be conferred by MADV infection. We provide evidence of persistent neurologic symptoms up to 5 years following MADV and VEEV exposure.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: found
Neurological Sequelae Resulting from Encephalitic Alphavirus Infection
- Record: found
- Abstract: found
- Article: not found
Eastern equine encephalitis in Latin America.
- Record: found
- Abstract: found
- Article: not found