- Record: found
- Abstract: found
- Article: found
Cpt1a gene expression in peripheral blood mononuclear cells as an early biomarker of diet-related metabolic alterations
Read this article at
Abstract
Background
Research on biomarkers that provide early information about the development of future metabolic alterations is an emerging discipline. Gene expression analysis in peripheral blood mononuclear cells (PBMC) is a promising tool to identify subjects at risk of developing diet-related diseases.
Objective
We analysed PBMC expression of key energy homeostasis-related genes in a time-course analysis in order to find out early markers of metabolic alterations due to sustained intake of high-fat (HF) and high-protein (HP) diets.
Design
We administered HF and HP diets (4 months) to adult Wistar rats in isocaloric conditions to a control diet, mainly to avoid overweight associated with the intake of hyperlipidic diets and, thus, to be able to characterise markers of metabolically obese normal-weight (MONW) syndrome. PBMC samples were collected at different time points of dietary treatment and expression of relevant energy homeostatic genes analysed by real-time reverse transcription-polymerase chain reaction. Serum parameters related with metabolic syndrome, as well as fat deposition in liver, were also analysed.
Results
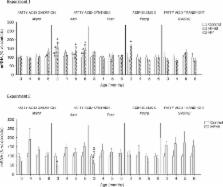
The most outstanding results were those obtained for the expression of the lipolytic gene carnitine palmitoyltransferase 1a ( Cpt1a). Cpt1a expression in PBMC increased after only 1 month of exposure to both unbalanced diets, and this increased expression was maintained thereafter. Interestingly, in the case of the HF diet, Cpt1a expression was altered even in the absence of increased body weight but correlated with alterations such as higher insulin resistance, alteration of serum lipid profile and, particularly, increased fat deposition in liver, a feature characteristic of metabolic syndrome, which was even observed in animals fed with HP diet.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Dietary protein intake and human health.
- Record: found
- Abstract: found
- Article: not found
Pathways and control of ketone body metabolism: on the fringe of lipid biochemistry.
- Record: found
- Abstract: found
- Article: not found