- Record: found
- Abstract: found
- Article: found
An essential thioredoxin-type protein of Trypanosoma brucei acts as redox-regulated mitochondrial chaperone
Read this article at
Abstract
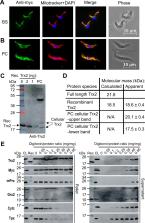
Most known thioredoxin-type proteins (Trx) participate in redox pathways, using two highly conserved cysteine residues to catalyze thiol-disulfide exchange reactions. Here we demonstrate that the so far unexplored Trx2 from African trypanosomes ( Trypanosoma brucei) lacks protein disulfide reductase activity but functions as an effective temperature-activated and redox-regulated chaperone. Immunofluorescence microscopy and fractionated cell lysis revealed that Trx2 is located in the mitochondrion of the parasite. RNA-interference and gene knock-out approaches showed that depletion of Trx2 impairs growth of both mammalian bloodstream and insect stage procyclic parasites. Procyclic cells lacking Trx2 stop proliferation under standard culture conditions at 27°C and are unable to survive prolonged exposure to 37°C, indicating that Trx2 plays a vital role that becomes augmented under heat stress. Moreover, we found that Trx2 contributes to the in vivo infectivity of T. brucei. Remarkably, a Trx2 version, in which all five cysteines were replaced by serine residues, complements for the wildtype protein in conditional knock-out cells and confers parasite infectivity in the mouse model. Characterization of the recombinant protein revealed that Trx2 can coordinate an iron sulfur cluster and is highly sensitive towards spontaneous oxidation. Moreover, we discovered that both wildtype and mutant Trx2 protect other proteins against thermal aggregation and preserve their ability to refold upon return to non-stress conditions. Activation of the chaperone function of Trx2 appears to be triggered by temperature-mediated structural changes and inhibited by oxidative disulfide bond formation. Our studies indicate that Trx2 acts as a novel chaperone in the unique single mitochondrion of T. brucei and reveal a new perspective regarding the physiological function of thioredoxin-type proteins in trypanosomes.
Author summary
African trypanosomes are the causative agents of human sleeping sickness and Nagana cattle disease. These strictly extracellular pathogens multiply in the blood and body fluids of their mammalian hosts and the tsetse fly vector, where efficient redox regulation is essential for parasite survival. While most organisms use the glutathione/glutathione reductase and thioredoxin/thioredoxin reductase couples to maintain cellular redox balance, trypanosomes rely on a unique trypanothione-based thiol metabolism to survive exogenous and endogenous oxidative stresses. Despite the lack of thioredoxin reductases, the Trypanosoma brucei genome encodes thioredoxins, raising questions for their biological function. Our work is the first report on T. brucei thioredoxin-2 (Trx2). We show that Trx2 is located in the mitochondrion and its absence affects parasite proliferation and infectivity. Recombinant Trx2 lacks protein disulfide reductase activity but protects proteins against aggregation and maintains them folding-competent. Remarkably, a mutant that is devoid of any cysteine residues is able to fully substitute for the authentic protein under in vitro and in vivo conditions. Our data reveal that Trx2 does not function as a classical thioredoxin but acts as a chaperone that plays a crucial role in the mitochondrion of T. brucei.
Related collections
Most cited references70
- Record: found
- Abstract: found
- Article: not found
Physiological functions of thioredoxin and thioredoxin reductase.
- Record: found
- Abstract: found
- Article: not found
High-throughput phenotyping using parallel sequencing of RNA interference targets in the African trypanosome.
- Record: found
- Abstract: found
- Article: not found