- Record: found
- Abstract: found
- Article: not found
Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers
Read this article at
- open (via free pdf)
- oa repository (via OAI-PMH doi match)
- oa repository (via OAI-PMH title and first author match)
- oa repository (via OAI-PMH title and first author match)
- oa repository (via OAI-PMH title and first author match)
- oa repository (via OAI-PMH title and first author match)
- oa repository (via pmcid lookup)
Powered by 
Abstract
Background
The relationship between the presence of antibodies to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the risk of subsequent reinfection remains unclear.
Methods
We investigated the incidence of SARS-CoV-2 infection confirmed by polymerase chain reaction (PCR) in seropositive and seronegative health care workers attending testing of asymptomatic and symptomatic staff at Oxford University Hospitals in the United Kingdom. Baseline antibody status was determined by anti-spike (primary analysis) and anti-nucleocapsid IgG assays, and staff members were followed for up to 31 weeks. We estimated the relative incidence of PCR-positive test results and new symptomatic infection according to antibody status, adjusting for age, participant-reported gender, and changes in incidence over time.
Results
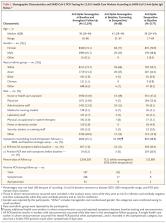
A total of 12,541 health care workers participated and had anti-spike IgG measured; 11,364 were followed up after negative antibody results and 1265 after positive results, including 88 in whom seroconversion occurred during follow-up. A total of 223 anti-spike–seronegative health care workers had a positive PCR test (1.09 per 10,000 days at risk), 100 during screening while they were asymptomatic and 123 while symptomatic, whereas 2 anti-spike–seropositive health care workers had a positive PCR test (0.13 per 10,000 days at risk), and both workers were asymptomatic when tested (adjusted incidence rate ratio, 0.11; 95% confidence interval, 0.03 to 0.44; P=0.002). There were no symptomatic infections in workers with anti-spike antibodies. Rate ratios were similar when the anti-nucleocapsid IgG assay was used alone or in combination with the anti-spike IgG assay to determine baseline status.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study
- Record: found
- Abstract: found
- Article: not found
Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections
- Record: found
- Abstract: found
- Article: not found